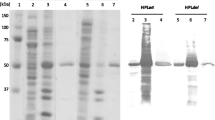
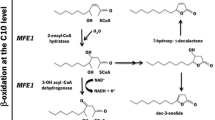
Abstract
Green leaf volatiles impart characteristic aroma and flavour to a variety of natural foods due to their inherent grassy note contributed by aldehydes. Hydroperoxide lyase (HPL) is an enzyme that helps in the cleavage of fatty acid hydroperoxides to short-chain aldehydes and ω-oxo-acids. A tomato hydroperoxide lyase gene was successfully expressed in E. coli BL21 (DE3) cells and used in the subsequent production of (Z)-3-hexenal. Biochemical characterization of the HPL activity exhibited by these whole cells enabled the development of a suitable one-pot reaction process for conversion of the hydroperoxide substrate to the corresponding aldehyde, (Z)-3-hexenal, and finally to (Z)-3-hexenol, a high-value flavour and fragrance ingredient.







Similar content being viewed by others
References
Atwal AS, Bisakowski B, Richard S, Robert N and Lee B 2005 Cloning and secretion of tomato hydroperoxide lyase in Pichia pastoris. Process Biochem. 40 95–102
Bate NJ, Sivasankar S, Moxon C, Riley John MC, Thompson JE and Rothstein SJ 1998 Molecular characterization of an Arabidopsis gene encoding hydroperoxide lyase, a cytochrome P-450 that is wound inducible. Plant Physiol. 117 1393–1400
Brühlmann F and Bosijokovic B 2016 Efficient biochemical cascade for accessing green leaf alcohols. Org. Process Res. Dev. 20 1974–1978
Buchhaupt M, Guder JC, Etschmann MMW and Schrader J 2012 Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl. Microbiol. Biotechnol. 93 159–168
Fauconnier ML, Perez AG, Sanz C and Marlier M 1997 Purification and characterization of tomato leaf (Lycopersicon esculentum Mill.) hydroperoxide lyase. J. Agric. Food Chem. 45 4232–4236
Froehlich JE, Itoh A and Howe GA 2001 Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s iInvolved in oxylipin metabolism are targeted to different membranes of chloroplast envelope. Plant Physiol. 125 306–331
Gigot C, Ongena M, Fauconnier ML, et al. 2012 Optimization and scaling up of a biotechnological synthesis of natural green leaf volatiles using Beta vulgaris hydroperoxide lyase. Process Biochem. 47 2547–2551
Hausler A, Lerch K, Muheim A and Silke N 2001 Hydroperoxide lyases (US 6238898 B1)
Huang S, Li R, Zhang Z, et al. 2009 The genome of the cucumber, Cucumis sativus L. Nat. Genet. 41 1275–1281
Jacopini S, Mariani M, de Caraffa VB-B, et al. 2016 Olive recombinant hydroperoxide lyase, an efficient biocatalyst for synthesis of green leaf volatiles. Appl. Biochem. Biotechnol. 179 671–683
Kim IS and Grosch W 1981 Partial purification and properties of a hydroperoxide lyase from fruits of pear 29 1220–1225
Kumar S, Stecher G, Li M, Knyaz C and Tamura K 2018 MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35 1547–1549
Matsui K, Fukutomi S, Wilkinson J, et al. 2001 Effect of Overexpression of fatty acid 9-hydroperoxide lyase in tomatoes (Lycopersicon esculentum Mill.). J. Agric. Food Chem. 49 5418–5424
Matsui K, Minami A, Hornung E, et al. 2006 Biosynthesis of fatty acid derived aldehydes is induced upon mechanical wounding and its products show fungicidal activities in cucumber. Phytochemistry 67 649–657
Matsui K, Miyahara, Wilkinson J, et al. 2000a Fatty acid hydroperoxide lyase in tomato fruits: Cloning and properties of a recombinant enzyme expressed in Escherichia coli. Biosci. Biotechnol. Biochem. 64 1189–1196
Matsui K, Toyota H, Kajiwara T, Kakuno T and Hatanaka A 1991 Fatty acid hydroperoxide cleaving enzyme hydroperoxide lyase from tea leaves. Phytochemistry 30 2109–2113
Matsui K, Ujita C, Fujimoto S, et al. 2000b Fatty acid 9- and 13-hydroperoxide lyases from cucumber. FEBS Lett. 481 183–188
Mu W, Xue Q, Jiang B and Hua Y 2012 Molecular cloning, expression, and enzymatic characterization of Solanum tuberosum hydroperoxide lyase. Eur. Food Res. Technol. 234 723–731
Noordermeer MA, Veldink GA and Vliegenthart JFG 1999 Alfalfa contains substantial 9-hydroperoxide lyase activity and a 3 Z:2 E-enal isomerase. FEBS Lett. 443 201–204
Noordermeer MA, van der Goot W, van Kooij AJ, et al. 2002 Development of a biocatalytic process for the production of C6-aldehydes from vegetable oils by soybean lipoxygenase and recombinant hydroperoxide lyase. J. Agric. Food Chem. 50 4270–4274
Olias JM, Rios JJ, Valle M, et al. 1990 Fatty acid hydroperoxide lyase in germinating soybean seedlings. J. Agric. Food Chem. 38 624–630
Ono E, Handa T, Koeduka T, et al. 2016 CYP74B24 is the 13-hydroperoxide lyase involved in biosynthesis of green leaf volatiles in tea (Camellia sinensis). Plant Physiol. Biochem. 98 112–118
Perez AG, Sanz C, Olias R and Olias JM 1999 Lipoxygenase and hydroperoxide lyase activities in ripening strawberry fruits. J. Agric. Food Chem. 47 249–253
Rabetafika HN, Gigot C, Fauconnier M-L, et al. 2008 Sugar beet leaves as new source of hydroperoxide lyase in a bioprocess producing green-note aldehydes. Biotechnol. Lett. 30 1115–1119
Robert X and Gouet P 2014 Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 42 W320–W324
Schreier P and Lorenz G 1982 Separation, partial purification and characterization of a fatty acid hydroperoxide cleaving enzyme from apple and tomato fruits. Z. Naturforsch. C 37 165–173
Shibata Y, Matsui K, Kajiwara T and Hatanaka A 1995 Purification and properties of fatty acid hydroperoxide lyase from green bell pepper fruits. Plant Cell Physiol. 36 147–156
Shimadzu Application Note 2020 Modifying AOAC method 996.06 for FAME analysis in foods: Faster throughput using hydrogen carrier gas
Stolterfoht H, Rinnofner C, Winkler M and Pichler H 2019 Recombinant lipoxygenases and hydroperoxide lyases for the synthesis of green leaf volatiles. J. Agric. Food Chem. 67 13367–13392
Suurmeijer CN, Pérez-Gilabert M, van Unen DJ, et al. 2000 Purification, stabilization and characterization of tomato fatty acid hydroperoxide lyase. Phytochemistry 53 177–185
Thompson JD, Higgins DG, Gibson TJ 1994 CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680
Tijet N, Wäspi U, Gaskin DJH, et al. 2000 Purification, molecular cloning, and expression of the gene encoding fatty acid 13-hydroperoxide lyase from guava fruit (Psidium guajava). Lipids 35 709–720
Tijet N, Schneider C, Muller BL and Brash AR 2001 Biogenesis of volatile aldehydes from fatty acid hydroperoxides: Molecular cloning of a hydroperoxide lyase (CYP74C) with specificity for both the 9- and 13-hydroperoxides of linoleic and linolenic acids. Arch. Biochem. Biophys. 386 281–289
Vick BA and Zimmerman DC 1976 Lipoxygenase and hydroperoxide lyase in germinating watermelon seedlings. Plant Physiol. 57 780–788
Wan XH, Chen SX, Wang CY, et al. 2013 Isolation, expression, and characterization of a hydroperoxide lyase gene from cucumber. Int. J. Mol. Sci. 14 22082–22101
Acknowledgements
This work was completely funded by Tojo Vikas International, New Delhi, India, and the authors wish to thank them for their support. The authors wish to specially thank the Bangalore Bioinnovation Centre for the infrastructure support provided towards completion of all experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by Rajesh Vishwanathan.
Corresponding editor: Rajesh Vishwanathan
Rights and permissions
About this article
Cite this article
Kaur, I., Korrapati, N., Bonello, J. et al. Biosynthesis of natural aroma compounds using recombinant whole-cell tomato hydroperoxide lyase biocatalyst. J Biosci 47, 37 (2022). https://doi.org/10.1007/s12038-022-00269-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12038-022-00269-4