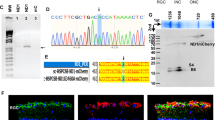
Abstract
Mitochondrial dysfunction mediated loss of respiration, oxidative stress, and loss of cellular homeostasis contributes to the neuronal and axonal degenerations permanent loss of function in experimental autoimmune encephalomyelitis model (EAE) of multiple sclerosis (MS). To address the mitochondrial dysfunction mediated visual loss in EAE mice, self-complementary adeno-associated virus (scAAV) containing the NADH-dehydrogenase type-2 (NDI1) complex I gene was intravitreally injected into the mice after the onset of visual defects. Visual function assessed by pattern electroretinogram (PERGs) showed progressive loss of function in EAE mice were improved significantly in NDI1 gene therapy-treated mice. Serial optical coherence tomography (OCT) revealed that progressive thinning of inner retinal layers in EAE mice was prevented upon NDI1 expression. The 45% optic nerve axonal and 33% retinal ganglion cell (RGC) loss contributed to the permanent loss of visual function in EAE mice were ameliorated by NDI1-mediated prevention of mitochondrial cristae dissolution and improved mitochondrial homeostasis. In conclusion, targeting the dysfunctional complex I using NDI1 gene can be an approach to address axonal and neuronal loss responsible for permanent disability in MS that is unaltered by current disease modifying drugs.








Similar content being viewed by others
References
Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, Jayandharan GR, Ling C et al (2010) High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol.Ther. 18:2048
Coleman MP, Perry VH (2002) Axon pathology in neurological disease: a neglected therapeutic target. Trends Neurosci 25:532
Toosy AT, Mason DF, Miller DH (2014) Optic neuritis. Lancet Neurol 13:83
Aktas O, Albrecht P, Hartung HP (2016) Optic neuritis as a phase 2 paradigm for neuroprotection therapies of multiple sclerosis: update on current trials and perspectives. Curr.Opin.Neurol. 29:199
Shirani A, Zhao Y, Karim ME, Evans C, Kingwell E, van der Kop ML, Oger J, Gustafson P et al (2012) Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA 308:247
Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD et al (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N.Engl.J.Med 354:899
Noseworthy JH (2003) Management of multiple sclerosis: current trials and future options. Curr.Opin.Neurol. 16:289
Benarroch EE (2008) N-acetylaspartate and N-acetylaspartylglutamate: neurobiology and clinical significance. Neurology 70:1353
Nikic I, Merkler D, Sorbara C, Brinkoetter M, Kreutzfeldt M, Bareyre FM, Bruck W, Bishop D et al (2011) A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat.Med. 17:495
Schattling B, Steinbach K, Thies E, Kruse M, Menigoz A, Ufer F, Flockerzi V, Bruck W et al (2012) TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat.Med
Kalman B (2006) Role of mitochondria in multiple sclerosis. Curr.Neurol.Neurosci.Rep. 6:244
Kalman B, Leist TP (2003) A mitochondrial component of neurodegeneration in multiple sclerosis. Neuromolecular.Med. 3:147
Kalman B, Alder H (1998) Is the mitochondrial DNA involved in determining susceptibility to multiple sclerosis? Acta NeurolScand 98:232
Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, Gudz T, Macklin WB et al (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann.Neurol. 59:478
Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J (2006) Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J.Biol.Chem. 281:31950
Talla V, Koilkonda R, Porciatti V, Chiodo V, Boye SL, Hauswirth WW, Guy J (2015) Complex I subunit gene therapy with NDUFA6 ameliorates neurodegeneration in EAE. Invest Ophthalmol.Vis.Sci. 56:1129
Talla V, Porciatti V, Chiodo V, Boye SL, Hauswirth WW, Guy J (2014) Gene therapy with mitochondrial heat shock protein 70 suppresses visual loss and optic atrophy in experimental autoimmune encephalomyelitis. Invest OphthalmolVisSci 55:5214
Talla V, Yu H, Chou TH, Porciatti V, Chiodo V, Boye SL, Hauswirth WW, Lewin AS et al (2013) NADH-dehydrogenase type-2 suppresses irreversible visual loss and Neurodegeneration in the EAE animal model of MS. Mol.Ther
Wallace DC, Singh G, Lott MT, Hodge JA, Schurr TG, Lezza AM, Elsas LJ, Nikoskelainen EK (1988) Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science 242:1427
Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson's disease. J.Neurochem. 55:2142
Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex I deficiency in Parkinson's disease. J.Neurochem. 54:823
Iwata M, Lee Y, Yamashita T, Yagi T, Iwata S, Cameron AD, Maher MJ (2012) The structure of the yeast NADH dehydrogenase (Ndi1) reveals overlapping binding sites for water- and lipid-soluble substrates. Proc.Natl.Acad.Sci.U.S.A 109:15247
Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N (2000) Production and purification of recombinant adeno-associated virus. Methods Enzymol 316:743
Legland D, Arganda-Carreras I, Andrey P (2016) MorphoLibJ: integrated library and plugins for mathematical morphology with ImageJ. Bioinformatics. 32:3532
Bjartmar C, Trapp BD (2001) Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr.Opin.Neurol. 14:271
Silber E, Sharief MK (1999) Axonal degeneration in the pathogenesis of multiple sclerosis. J.Neurol.Sci. 170:11
Banitt MR, Ventura LM, Feuer WJ, Savatovsky E, Luna G, Shif O, Bosse B, Porciatti V (2013) Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol.Vis.Sci. 54:2346
Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, Vetter ML, Marsh-Armstrong N et al (2008) Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J.Neurosci. 28:2735
Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, Lassmann H, Turnbull DM et al (2011) Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann.Neurol. 69:481
Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, Kalman B (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J.Neurol.Sci. 177:95
Witte ME, Bo L, Rodenburg RJ, Belien JA, Musters R, Hazes T, Wintjes LT, Smeitink JA et al (2009) Enhanced number and activity of mitochondria in multiple sclerosis lesions. J.Pathol. 219:193
Kumleh HH, Riazi GH, Houshmand M, Sanati MH, Gharagozli K, Shafa M (2006) Complex I deficiency in Persian multiple sclerosis patients. J.Neurol.Sci
Bosley TM, Constantinescu CS, Tench CR, bu-Amero KK (2007) Mitochondrial changes in leukocytes of patients with optic neuritis. Mol.Vis. 13:1516
Seo BB, Kitajima-Ihara T, Chan EK, Scheffler IE, Matsuno-Yagi A, Yagi T (1998) Molecular remedy of complex I defects: rotenone-insensitive internal NADH-quinone oxidoreductase of Saccharomyces cerevisiae mitochondria restores the NADH oxidase activity of complex I-deficient mammalian cells. Proc.Natl.Acad.Sci.U.S.A 95:9167
Seo BB, Wang J, Flotte TR, Yagi T, Matsuno-Yagi A (2000) Use of the NADH-quinone oxidoreductase (NDI1) gene of Saccharomyces cerevisiae as a possible cure for complex I defects in human cells. J.Biol.Chem. 275:37774
Bai Y, Hajek P, Chomyn A, Chan E, Seo BB, Matsuno-Yagi A, Yagi T, Attardi G (2001) Lack of complex I activity in human cells carrying a mutation in MtDNA- encoded ND4 subunit is corrected by the Saccharomyces cerevisiae NADH- Quinone Oxidoreductase (NDI1) gene. J.Biol.Chem
Bahadorani S, Cho J, Lo T, Contreras H, Lawal HO, Krantz DE, Bradley TJ, Walker DW (2010) Neuronal expression of a single-subunit yeast NADH-ubiquinone oxidoreductase (Ndi1) extends Drosophila lifespan. Aging Cell 9:191
DeCorby A, Gaskova D, Sayles LC, Lemire BD (2007, 1767) Expression of Ndi1p, an alternative NADH:ubiquinone oxidoreductase, increases mitochondrial membrane potential in a C. elegans model of mitochondrial disease. Biochim.Biophys.Acta:1157
Marella M, Seo BB, Thomas BB, Matsuno-Yagi A, Yagi T (2010) Successful amelioration of mitochondrial optic neuropathy using the yeast NDI1 gene in a rat animal model. PLoS.One 5:e11472
Marella M, Seo BB, Flotte TR, Matsuno-Yagi A, Yagi T (2011) No immune responses by the expression of the yeast Ndi1 protein in rats. PLoS.One 6:e25910
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE et al (2008) Effect of gene therapy on visual function in Leber's congenital Amaurosis. N.Engl.J.Med
Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL et al (2009) Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: A phase 1 dose-escalation trial. Lancet 374:1597
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA et al (2008) Safety and efficacy of gene transfer for Leber's congenital amaurosis. N.Engl.J.Med 358:2240
Hauswirth WW, Aleman TS, Kaushal S, Cideciyan AV, Schwartz SB, Wang L, Conlon TJ, Boye SL et al (2008) Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. HumGene Ther 19:979
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K et al (2006) Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat.Med. 12:342
Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV et al (2003) AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101:2963
Flotte TR, Laube BL (2001) Gene therapy in cystic fibrosis. Chest 120:124S
Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D et al (2007) Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet 369:2097
Sergott RC (2005) Optical coherence tomography: measuring in-vivo axonal survival and neuroprotection in multiple sclerosis and optic neuritis. Curr.Opin.Ophthalmol. 16:346
Koilkonda R, Yu H, Talla V, Porciatti V, Feuer WJ, Hauswirth WW, Chiodo V, Erger KE et al (2014) LHON gene therapy vector prevents visual loss and optic neuropathy induced by G11778A mutant mitochondrial DNA: Biodistribution and toxicology profile. Invest Ophthalmol.Vis.Sci. 55:7739
Dayton RD, Wang DB, Klein RL (2012) The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert.Opin.Biol.Ther. 12:757
Duque S, Joussemet B, Riviere C, Marais T, Dubreil L, Douar AM, Fyfe J, Moullier P et al (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol.Ther. 17:1187
Acknowledgments
The authors thank Dr. Vittorio Porciatti for his advice on PERG experiments and Dr. William W. Hauswirth’s lab for assistance with AAV viral packaging.
Funding
This work was partially supported by an unrestricted grant to Bascom Palmer Eye institute from Research to Prevent Blindness, NIH core grant no. P30 EY014801.
Author information
Authors and Affiliations
Contributions
VT and RDK participated in conducting experiments, acquiring, analyzing, and interpreting data. VT drafted the manuscript with critical review from RDK and JG. JG and VT conceived and supervised the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
The protocol for the study was approved by the University of Miami Institutional Animal Care and Use Committee, and all the procedures were conducted in accordance with the United States Public Health Service’s Policy on Humane Care and Use of Laboratory Animals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 132 kb)
Rights and permissions
About this article
Cite this article
Talla, V., Koilkonda, R. & Guy, J. Gene Therapy with Single-Subunit Yeast NADH-Ubiquinone Oxidoreductase (NDI1) Improves the Visual Function in Experimental Autoimmune Encephalomyelitis (EAE) Mice Model of Multiple Sclerosis (MS). Mol Neurobiol 57, 1952–1965 (2020). https://doi.org/10.1007/s12035-019-01857-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-01857-6