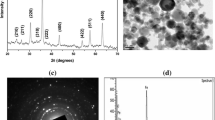
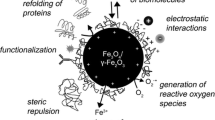
Abstract
Various iron-oxide nanoparticles have been in use for a long time as therapeutic and imaging agents and for supplemental delivery in cases of iron-deficiency. While all of these products have a specified size range of ∼40 nm and above, efforts are underway to produce smaller particles, down to ∼1 nm. Here, we show that after a 24-h exposure of SHSY-5Y human neuroblastoma cells to 10 μg/ml of 10 and 30 nm ferric oxide nanoparticles (Fe-NPs), cellular dopamine content was depleted by 68 and 52 %, respectively. Increases in activated tyrosine kinase c-Abl, a molecular switch induced by oxidative stress, and neuronal α-synuclein expression, a protein marker associated with neuronal injury, were also observed (55 and 38 % percent increases, respectively). Inhibition of cell-proliferation, significant reductions in the number of active mitochondria, and a dose-dependent increase in reactive oxygen species (ROS) were observed in neuronal cells. Additionally, using a rat in vitro blood–brain barrier (BBB) model, a dose-dependent increase in ROS accompanied by increased fluorescein efflux demonstrated compromised BBB integrity. To assess translational implications, in vivo Fe-NP-induced neurotoxicity was determined using in vivo MRI and post-mortem neurochemical and neuropathological correlates in adult male rats after exposure to 50 mg/kg of 10 nm Fe-NPs. Significant decrease in T 2 values was observed. Dynamic observations suggested transfer and retention of Fe-NPs from brain vasculature into brain ventricles. A significant decrease in striatal dopamine and its metabolites was also observed, and neuropathological correlates provided additional evidence of significant nerve cell body and dopaminergic terminal damage as well as damage to neuronal vasculature after exposure to 10 nm Fe-NPs. These data demonstrate a neurotoxic potential of very small size iron nanoparticles and suggest that use of these ferric oxide nanoparticles may result in neurotoxicity, thereby limiting their clinical application.






Similar content being viewed by others
References
Hood E (2004) Nanotechnology: looking as we leap. Environ Health Perspect 112(13):A740–749
Wang B, Feng WY, Wang M, Shi JW, Zhang F, Ouyang H, Zhao YL, Chai ZF et al (2007) Transport of intranasally instilled fine Fe2O3 particles into the brain: micro-distribution, chemical states, and histopathological observation. Biol Trace Elem Res 118(3):233–243. doi:10.1007/s12011-007-0028-6
Jain TK, Reddy MK, Morales MA, Leslie-Pelecky DL, Labhasetwar V (2008) Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharm 5(2):316–327. doi:10.1021/mp7001285
Mehta SH, Webb RC, Ergul A, Tawfik A, Dorrance AM (2004) Neuroprotection by tempol in a model of iron-induced oxidative stress in acute ischemic stroke. Am J Physiol Regul Integr Comp Physiol 286(2):R283–288. doi:10.1152/ajpregu.00446.2002
Karmakar A, Zhang Q, Zhang Y (2014) Neurotoxicity of nanoscale materials. J Food Drug Anal 22(1):147–160. doi:10.1016/j.jfda.2014.01.012
Simonian NA, Coyle JT (1996) Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 36:83–106. doi:10.1146/annurev.pa.36.040196.000503
Imam SZ, Ali SF (2000) Selenium, an antioxidant, attenuates methamphetamine-induced dopaminergic toxicity and peroxynitrite generation. Brain Res 855(1):186–191
Imam SZ, Islam F, Itzhak Y, Slikker W Jr, Ali SF (2000) Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Ann N Y Acad Sci 914:157–171
Imam SZ, Itzhak Y, Cadet JL, Islam F, Slikker W Jr, Ali SF (2001) Methamphetamine-induced alteration in striatal p53 and bcl-2 expressions in mice. Brain Res Mol Brain Res 91(1–2):174–178
Imam SZ, Newport GD, Islam F, Slikker W Jr, Ali SF (1999) Selenium, an antioxidant, protects against methamphetamine-induced dopaminergic neurotoxicity. Brain Res 818(2):575–578
Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W Jr, Ali SF (2001) Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem 76(3):745–749
Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM et al (1999) Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med 5(12):1403–1409. doi:10.1038/70978
Binienda ZK, Beaudoin MA, Gough B, Ali SF, Virmani A (2010) Assessment of 3-nitropropionic acid-evoked peripheral neuropathy in rats: neuroprotective effects of acetyl-l-carnitine and resveratrol. Neurosci Lett 480(2):117–121. doi:10.1016/j.neulet.2010.06.020
Pisanic TR 2nd, Blackwell JD, Shubayev VI, Finones RR, Jin S (2007) Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28(16):2572–2581. doi:10.1016/j.biomaterials.2007.01.043
Wu J, Ding T, Sun J (2013) Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology 34:243–253. doi:10.1016/j.neuro.2012.09.006
Xue Y, Wu J, Sun J (2012) Four types of inorganic nanoparticles stimulate the inflammatory reaction in brain microglia and damage neurons in vitro. Toxicol Lett 214(2):91–98. doi:10.1016/j.toxlet.2012.08.009
Zhang Y, Ferguson SA, Watanabe F, Jones Y, Xu Y, Biris AS, Hussain S, Ali SF (2013) Silver nanoparticles decrease body weight and locomotor activity in adult male rats. Small 9(9–10):1715–1720. doi:10.1002/smll.201201548
Imam SZ, Zhou Q, Yamamoto A, Valente AJ, Ali SF, Bains M, Roberts JL, Kahle PJ et al (2011) Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson’s disease. J Neurosci 31(1):157–163. doi:10.1523/JNEUROSCI.1833-10.2011
Wang H, Joseph JA (1999) Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med 27(5–6):612–616
Trickler WJ, Lantz SM, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Paule MG, Slikker W et al (2012) Effects of copper nanoparticles on rat cerebral microvessel endothelial cells. Nanomedicine (Lond) 7(6):835–846. doi:10.2217/nnm.11.154
Trickler WJ, Lantz SM, Murdock RC, Schrand AM, Robinson BL, Newport GD, Schlager JJ, Oldenburg SJ et al (2010) Silver nanoparticle induced blood–brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol Sci 118(1):160–170. doi:10.1093/toxsci/kfq244
Deng XL, He F, Peng J, Yang LF, Zhang CL, Xiang QL, Wu LW, Wang GL et al (2011) Influence of lipopolysaccharide on the permeability of rat brain microvascular endothelial cells and the molecular mechanism. Zhongguo Dang Dai Er Ke Za Zhi 13(11):908–911
Imam SZ, Trickler W, Kimura S, Binienda ZK, Paule MG, Slikker W Jr, Li S, Clark RA et al (2013) Neuroprotective efficacy of a new brain-penetrating C-Abl inhibitor in a murine parkinson’s disease model. PLoS One 8(5), e65129. doi:10.1371/journal.pone.0065129
Colvin VL (2003) The potential environmental impact of engineered nanomaterials. Nat Biotechnol 21(10):1166–1170. doi:10.1038/nbt875
Borm PJ, Muller-Schulte D (2006) Nanoparticles in drug delivery and environmental exposure: same size, same risks? Nanomedicine (Lond) 1(2):235–249. doi:10.2217/17435889.1.2.235
Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S (2006) Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci 90(1):23–32. doi:10.1093/toxsci/kfj084
Lewinski N, Colvin V, Drezek R (2008) Cytotoxicity of nanoparticles. Small 4(1):26–49. doi:10.1002/smll.200700595
Nel A, Xia T, Madler L, Li N (2006) Toxic potential of materials at the nanolevel. Science 311(5761):622–627. doi:10.1126/science.1114397
Xia T, Kovochich M, Brant J, Hotze M, Sempf J, Oberley T, Sioutas C, Yeh JI et al (2006) Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett 6(8):1794–1807. doi:10.1021/nl061025k
Sharma HS, Hussain S, Schlager J, Ali SF, Sharma A (2010) Influence of nanoparticles on blood–brain barrier permeability and brain edema formation in rats. Acta Neurochir Suppl 106:359–364. doi:10.1007/978-3-211-98811-4_65
Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N et al (2008) Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood–brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36(2):289–310. doi:10.1177/0192623307313011
Horn D, Fontanesi F, Barrientos A (2008) Exploring protein-protein interactions involving newly synthesized mitochondrial DNA-encoded proteins. Methods Mol Biol 457:125–139
Horn D, Al-Ali H, Barrientos A (2008) Cmc1p is a conserved mitochondrial twin CX9C protein involved in cytochrome c oxidase biogenesis. Mol Cell Biol 28(13):4354–4364. doi:10.1128/MCB.01920-07
Charles AL, Guilbert AS, Bouitbir J, Goette-Di Marco P, Enache I, Zoll J, Piquard F, Geny B (2011) Effect of postconditioning on mitochondrial dysfunction in experimental aortic cross-clamping. Br J Surg 98(4):511–516. doi:10.1002/bjs.7384
Ali SF, Binienda ZK, Imam SZ (2011) Molecular aspects of dopaminergic neurodegeneration: gene-environment interaction in parkin dysfunction. Int J Environ Res Public Health 8(12):4702–4713. doi:10.3390/ijerph8124702
Zhu MT, Wang Y, Feng WY, Wang B, Wang M, Ouyang H, Chai ZF (2010) Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol 10(12):8584–8590
Karlsson HL, Cronholm P, Gustafsson J, Moller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21(9):1726–1732. doi:10.1021/tx800064j
Earnshaw WC, Martins LM, Kaufmann SH (1999) Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68:383–424. doi:10.1146/annurev.biochem.68.1.383
Earnshaw WC (1999) Apoptosis. A cellular poison cupboard. Nature 397(6718):387–389. doi:10.1038/17015
Alarifi S, Ali D, Alkahtani S, Alhader MS (2014) Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line. Biol Trace Elem Res 159(1–3):416–424. doi:10.1007/s12011-014-9972-0
Alarifi S, Ali D, Alakhtani S, Al Suhaibani ES, Al-Qahtani AA (2014) Reactive oxygen species-mediated DNA damage and apoptosis in human skin epidermal cells after exposure to nickel nanoparticles. Biol Trace Elem Res 157(1):84–93. doi:10.1007/s12011-013-9871-9
Ahamed M, Alhadlaq HA, Alam J, Khan MA, Ali D, Alarafi S (2013) Iron oxide nanoparticle-induced oxidative stress and genotoxicity in human skin epithelial and lung epithelial cell lines. Curr Pharm Des 19(37):6681–6690
Thomsen LB, Linemann T, Pondman KM, Lichota J, Kim KS, Pieters RJ, Visser GM, Moos T (2013) Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem Neurosci 4(10):1352–1360. doi:10.1021/cn400093z
Morales M, Root DH (2014) Glutamate neurons within the midbrain dopamine regions. Neuroscience 282C:60–68. doi:10.1016/j.neuroscience.2014.05.032
Dringen R (2000) Glutathione metabolism and oxidative stress in neurodegeneration. Eur J Biochem 267(16):4903
Taranukhin AG, Taranukhina EY, Saransaari P, Podkletnova IM, Pelto-Huikko M, Oja SS (2010) Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J Biomed Sci 17(1):S12. doi:10.1186/1423-0127-17-S1-S12
Repetto MG, Ossani G, Monserrat AJ, Boveris A (2010) Oxidative damage: the biochemical mechanism of cellular injury and necrosis in choline deficiency. Exp Mol Pathol 88(1):143–149. doi:10.1016/j.yexmp.2009.11.002
van Domburg PH, ten Donkelaar HJ (1991) The human substantia nigra and ventral tegmental area. A neuroanatomical study with notes on aging and aging diseases. Adv Anat Embryol Cell Biol 121:1–132
Hastie T, Tibshirani R, Botstein D, Brown P (2001) Supervised harvesting of expression trees. Genome Biol 2 (1):RESEARCH0003
Disclaimer
The research reported here was funded by the NCTR/FDA protocol # E −07394.01. This document has been reviewed in accordance with US Food and Drug Administration (FDA) policy and approved for publication. Approval does not signify that the contents necessarily reflect the position or opinions of the FDA nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the FDA.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Susan M. Lantz-McPeak and Elvis Cuevas contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(A) Three optimal clusters of caspase 3/7 positive cells was determined via Euclidean squared GAP statistic using K-means from 1 to 10 [49]. Subsequent self-organizing map (SOM) in a 3 × 1 matrix (B) helped distinguish which data points fell into each cluster. Each of the four columns seen within each SOM group corresponds to the PCA data in the following columns: (a) percent of caspase positive cells, (b) mean area of all cells (c) mean area of all nuclei, and (d) mean caspase-positive stain intensity, respectively. In the SOM heat map, green indicates a positive response; with darker green signifying a less positive response Red indicates a negative response, with darker red signifying a less negative response. (PDF 447 kb)
ESM 2
(MOV 8828 kb)
Rights and permissions
About this article
Cite this article
Imam, S.Z., Lantz-McPeak, S.M., Cuevas, E. et al. Iron Oxide Nanoparticles Induce Dopaminergic Damage: In vitro Pathways and In Vivo Imaging Reveals Mechanism of Neuronal Damage. Mol Neurobiol 52, 913–926 (2015). https://doi.org/10.1007/s12035-015-9259-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9259-2