Abstract
Dysgeusia and nausea are common side effects observed in head and neck cancer patients treated with either exclusive radiotherapy or combined modality treatment. The aim of the present study was to prospectively evaluate dysgeusia, during treatment and follow-up, using the chemotherapy-induced taste alteration scale (CiTAS), a metrics based on 18-items exploring three dimensions (quantitative and qualitative changes in taste perception, and diet-related issues) identified through a four-factor analysis: decline in basic taste, discomfort, phantogeusia–parageusia, and general taste alterations. Moreover, we scored, according to Common Toxicity Criteria Adverse Events, nausea and other treatment-related toxicities. Since, ginger is traditionally used to prevent and/or treat nausea and vomiting, we prophylactically employed a ginger-based supplement named Naumix/Naugin (Gamfarma, Milan, Italy), to potentially mitigate both nausea and taste impairment. Using the CiTAS scale, we highlighted a progressive increase in all dysgeusia dimensions, peaking at the VII week of treatment and a subsequent partial late recovery. In particular, we observed a recovery for discomfort, phantogeusia–parageusia, and general taste alterations at 6 months. Grade 2 nausea, observed to be as low as 12.9% potentially due to the use of ginger, peaked at the III week of treatment. Finally, for patients experiencing nausea, the dysgeusia dimension of discomfort was also relevant.
Similar content being viewed by others
Introduction
Radiation therapy (RT) is a mainstay option in the combined modality treatment (CMT) approach to head and neck cancer (HNC) [1, 2]. New technologies consistently improved the therapeutic window in this setting, but toxicity profile is not negligible [3, 4].
Dysgeusia is a rather frequent side effect during treatment. It is generally described by patients as a bitter, metallic, salty, and/or unpleasant taste. Taste alterations and symptoms of discomfort may remarkably affect the patient’s daily living and potentially the compliance to treatment. Dysgeusia has a relevant impact on quality of life (QoL), compromising the patient nutritional status with pathological weight loss. Dysgeusia can be assessed by several subjective and objective methods, including validated questionnaires and scales, which do represent a simple and useful tool for clinical practice [5,6,7,8].
In the systematic review by Hovan et al. [5], the weighted prevalence of dysgeusia in patients treated with exclusive chemotherapy (CT) was 56.3%, for those treated with radiotherapy only was 66.5%, while for those undergoing combined modality treatment, the rate was 76%. Approximately, 15% of patients treated with RT continued to experience persistent dysgeusia after the completion of treatment [5]. Another frequent and distressing RT side effect is nausea, reported in up to 50–80% of patients [9]. Nausea (and vomiting) can lead to complications such as dehydration, electrolyte imbalance, and malnutrition [9, 10], especially when patient are also given CT [11]. This may negatively impact on the compliance to treatment and QoL.
Both quantitative and qualitative taste alterations can be observed during treatment, such as
(a) hypo-ageusia: decreased or lost ability to taste; (b) discomfort: symptoms or complaints related to taste alteration, such as nausea; (c) phantogeusia/parageusia: continuous abnormal taste (usually bitter or metallic); and (d) general taste alterations.
We prospectively assessed taste alterations in a cohort of patients undergoing RT or CMT employing the chemotherapy (CT)-induced taste alteration scale (CiTAS), a scale based on 18-items, as proposed by Kano et al. [6]. The validated Italian version of the CiTAS scale was used [7]. All patients were given, during treatment and follow-up, a food supplement oral spray based on extract of ginger, anise, and vitamin B6 as a prophylactic approach to prevent nausea and dysgeusia. Ginger (Zingiber officinale) is a spice traditionally used to treat nausea and vomiting, given its property to accelerate gastric emptying and stimulate gastric antral contractions [12]. Given this positive impact on preventing nausea, ginger can potentially limit at the same time the onset of dysgeusia.
Materials and methods
Between July 2016 and January 2018, a total of 31 HN cancer patients were treated with definitive or adjuvant RT using Volumetric Arc Therapy (VMAT). Patients were selected whenever the oral cavity was at least partially included in the treatment volumes. All patients were given, during treatment and follow-up, Naumix/Naugin (Gamfarma, Milan, Italy). Written informed consent was obtained from all patients. The review board of our Institution Hospital approved the present study. Patients either naïve for surgery or having received prior surgical procedures, including neck dissection, were selected.
Chemotherapy induced taste alterations scale
The validated Italian version of the CiTAS scale was employed [7]. It is a self-administered questionnaire made of 18 items exploring 3 different dimensions: quantitative changes in taste perception (1–6 items), qualitative changes in taste perception (7–12 items) and diet-related issues (13–18 items). These 3 dimensions were identified through factor analysis: decline in basic taste (2–6 items), discomfort (13–18 items), phantogeusia–parageusia (10–12 items), and general taste alterations (1, 7–9 items). CiTAS is made up of a 5-point Likert-type scale. The first six items are graded as follows: (1) no difficulties in taste perception, (2) slight taste impairment, (3) intermediate taste impairment, (4) very difficult taste perception, (5) inability in tasting. The items between 7 and 18 are graded as follows: (1) no, (2) slightly, (3) medium, (4) quite, (5) very. The score calculation is obtained by dividing the sum of all scores for each subscale into the number of the items placed in the corresponding subscale. The maximum score is 5 points, the minimum is 1. Patients were evaluated at baseline (Bs), every week of treatment and during follow-up at three adjunctive time points (1 week, 1 and 6 months after RT end).
Physician assessed toxicities
On a weekly basis, we scored, according to the Common Toxicity Criteria for Adverse Events 4.0-4.02 (CTCAE 4.0) criteria, the following toxicity endpoints: nausea, vomiting, mucositis, dysphagia, xerostomia, cutaneous toxicity, dysgeusia. These toxicities were evaluated at baseline, during treatment and during follow-up at 3 adjunctive time points (1 week, 1 and 6 months after RT end).
Pain evaluation (visual analog scale, VAS)
Pain was subjectively evaluated by patients employing a visual analog scale (VAS) which rated the experienced symptom on a scale going from 0 (no pain) to 10 (maximal experienced pain); VAS was evaluated at baseline, during treatment on a weekly basis and during follow-up (1 week, 1 and 6 months after RT end).
Treatment approaches
The clusters of patients undergoing upfront surgical excision were selected accounting for disease presentation and eventual neck involvement (surgery to the primary site with or without neck dissection). Definitive or adjuvant RT was delivered employing intensity-modulated radiotherapy (IMRT), with a volumetric approach (VMAT) on a 6MV Elekta linear accelerator (Elekta, Stockholm, Sweden). A ‘simultaneous integrated boost’ (SIB) strategy was adopted. Image-guided RT (IGRT) was employed with cone beam computed tomography for image-guidance. In the adjuvant setting (with RT given no longer than 8 weeks after surgery), the most frequent fractionation was 54–66 Gy given in 2 Gy daily fraction to the ‘high risk volume’ and 51–54.4 Gy in 1.7–1.65 Gy daily fractions to the ‘low risk volume’. The most common RT schedule for definitive treatments was 70 Gy/35 fractions (2 Gy daily) to the macroscopic disease, 63 Gy/35 fractions (1.8 Gy daily) to the ‘intermediate risk prophylactic volume’ and 54.25 Gy/35 fractions (1.55 Gy daily) to the ‘low risk volume.’ Certain patients underwent exclusive RT. For those referred to CMT, CT was given as an induction treatment employing 2–3 cycles of docetaxel 75 mg/m2 and cisplatin 75 mg/m2 intravenously on day 1 and 5-fluorouracil 1000 mg/m2 on days 1–4 with continuous infusion (TPF regimen) or employing 4 cycles of Carboplatin AUC6 + Paclitaxel 200 mg/m2 (3-h infusion). Concurrent CT was given as weekly (35–40 mg/m2 body surface area) or three-weekly cisplatin (100 mg/m2 body surface area); weekly carboplatin was given to frail patients. Cetuximab was also given.
Statistical analysis
All statistical analyses were carried out using Statistical Package for Social Sciences (SPSS), version 20.0. A descriptive analysis of all data was performed with report on mean values, percentages, and standard deviations. Since the Kolmogorov–Smirnov test demonstrated a non-Gaussian distribution of variables, nonparametric tests were used. The Kruskal–Wallis test was used to assess differences between groups in the mean of continuous variables. Furthermore, multiple comparison analysis was always performed by adopting Mann–Whitney U-test. A p value < 0.05 was considered statistically significant.
Results
Patient, tumor. and treatment characteristics
Between July 2016 and January 2018, a total of 31 HN cancer patients were treated with definitive or adjuvant RT using Volumetric Arc Therapy (VMAT). Patients were selected whenever the oral cavity was at least partially included in the treatment volumes. Detailed patient, tumor, and treatment characteristics are provided in Tables 1 and 2. On average, patients were aged 64.6 years (range 34–83), mostly males (61.2%). Most observed tumor sites were oropharynx (25.8%) and oral cavity (16.1%). The most reported histology was squamous cell carcinoma (77.4%). Patients with T1–T2 disease were 58% while those with T3–T4 accounted for 38.7%. One patient (3.3%) had an unknown primary tumor. Most of the patients had nodal involvement, with N1–N3 in 70.9%. Up to 38.7% of the cases underwent definitive RT while an adjuvant treatment was delivered to 61.3%. Duration of RT was 6 weeks for 64.5% of patients and 7 for 35.5%. Induction CT was administered in 5 patients (16%), mainly with TPF regimen. Concurrent CT was given to 58% of patients, mostly with weekly Cisplatin (25.8%) or weekly Carboplatin (25.8%). One patient (3.2%) was treated with Cetuximab. Five patients were missing the 6-month observation time point. Among them, 3 patients were lost during follow-up and 2 died.
CiTAS
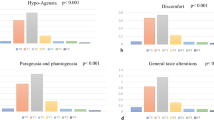
For detailed CiTAS results, see Table 3 and Fig. 1.
Mean hypo-ageusia score was 1.14 (± 0.4) at baseline. The score progressively increased during RT and reached the maximum values during the VII week at 2.82 (± 1.4), decreasing, after RT, down to 2.13 (± 1.23) at 1 month and to 1.21(± 0.20) at 6 months from the end of treatment. A statistically significant difference (p < 0.05) was observed, for Hypo-Ageusia, between baseline and first week of treatment. Moreover, a statistically significant difference was observed between the baseline and all the other weeks (II–VII weeks) of treatment, as with the I month observation time point (p < 0.01). A statistically significant difference (p < 0.05) was observed between the baseline and the VI month time point.
Similar data could be observed for discomfort score which was 1.14 (± 0.47) at baseline, increased up to 1.74 (± 0.98) at VII week and decreased at 1.27 (± 0.45) at 1 month and at 1.02 (± 0.08) at 6 months from the end of treatment. A statistically significant difference was observed between the baseline and all the other weeks (I–VII weeks) of treatment (p < 0.01) and, as well, with the I month observation time point (p < 0.05). No differences were observed between the baseline and last observational time point, 6 months from the end of RT.
The phantogeusia/parageusia score, which was 1.16 (± 0.60) at baseline, increased to 1.92 (± 1.08) at VII week and decreased to 1.36 (± 0.7) at 1 month and to 1.08 (± 0.27) 6 months after RT. A statistically significant difference (p < 0.05) was observed, in terms of phantogeusia/parageusia, between the baseline and the first 2 weeks of treatment. A statistical significant difference was observed between the baseline and all the other weeks (III–VII weeks) of treatment (p < 0.01). A statistically significant difference (p < 0.05) was observed between the baseline and the I month time point. No differences were observed between the baseline and last observational time point, 6 months from the end of RT.
A similar pattern was observed for the general taste alterations score: 0.17 (± 0.43) at baseline, 2.35 (± 1.04) at VII week of RT, 1.73 (± 0.87) after 1 month and 1.15 (± 0.14) at 6 months from RT end. A statistically significant difference (p < 0.05) was observed, in terms of general taste alteration, between the baseline and the first week of treatment. Moreover, a statistical significant difference was observed between the baseline and all the other weeks (II–VII weeks) of treatment together with the I month observation time point (p < 0.01). No differences were observed between the baseline and last observational time point, 6 months from the end of RT.
Physician toxicity analysis-CTCAE 4.0
Acute toxicities were generally mild. For details, see Table 4 (worst events). Regarding nausea (Fig. 2), Grade 1 was reported by 6.5% of patients at baseline, reaching the maximum value during the VI week (38.7%). No patient experienced nausea G2 at baseline; the peak of G2 nausea was observed at the III week (12.9% of patients), with optimal recovery using antiemetic drugs. We focused on the group of patients experiencing Grade 1–2 nausea (since no Grade 3 was recorded) and the mean values of CiTAS score. Interestingly, for patients experiencing G1–G2 nausea a significant correlation was found with the score of the dysgeusia dimension of discomfort (details in Table 5). This was observed from the II to the VI week of therapy (p < 0.05).
Among worst toxicity events, dysgeusia G2 was the most common toxicity described by the assessing physician during treatment and up to I month after RT. The second most common toxicity was grade 2 xerostomia, with the highest peak at VI (51.6%) and VII (60%) week. Grade 3 mucositis was observed at V (9.6%) and VI week (12.9%) of treatment. The worst toxicity event described at 6 month from the end of RT was xerostomia G2 (23%). The highest peak of G3 cutaneous toxicity was observed at the VI week (22.5%).
Physician toxicity analysis-visual analog scale, VAS (Fig. 3)
Two peaks were reported in the pain evaluation, regarding maximal experienced pain: at III week (mean value: 3.06) and VII week (mean value: 3.05).
Discussion
Patients affected with HNC are frequently treated with multimodality treatment and thus can experience a wide range of toxicities, acutely and lately. Dysgeusia and nausea are rather frequent side effects during therapy. The main clinical endpoint, prospectively evaluated in our study, was dysgeusia a common side effect for HNC patients during either exclusive RT or CMT [5]. Dysgeusia varies from the gradual loss of the taste sensation to an unpleasant distortion of several flavors. Many factors and other common toxicities are related to dysgeusia itself.
For instance, changes in the olfactory function, commonly observed during cancer therapies, are strictly linked to dysgeusia [5, 14]. As for dysgeusia, alterations in smell can be related also to the malignancy itself. The combination of these two alterations can affect the patient’s QoL leading to malnutrition, weight loss, and significant morbidity [15,16,17]. The oral mucosa cell damage, due to CT and RT, resulting in dysgeusia and alteration in olfaction, may occur through three different modalities: the progressive loss of normal receptor cells, the alteration in cell structure, and the interruption in neural coding [5].
Given the complexity of dysgeusia in terms of pathogenesis, it is important to use helpful tools, such as questionnaires and scales, to quantitate its clinical impact on patient’s daily living. Our study was aimed at describing the multidimensional pattern of dysgeusia.
We used the chemotherapy (CT)-induced taste alteration scale (CiTAS), a scale based on 18-items exploring three dimensions (quantitative and qualitative changes in taste perception, and diet-related issues) identified through a 4-factor analysis: decline in basic taste, discomfort, phantogeusia–parageusia, and general taste alterations. The questionnaire evaluation was performed at baseline, during treatment on a weekly basis and during observation (1 week, 1 and 6 months after RT end). A similar trend, for both quantitative and qualitative alterations, could be observed during therapy. A statistically significant increase in all dysgeusia dimension scores was reported during each week of treatment, compared to the baseline, with the highest peak at the VII week of RT. A partial recovery was highlighted after the end of RT, although a statistically significant difference was still present between the baseline and the first 2 observational time-point scores (1 week and 1 month after RT) for all dysgeusia dimensions. Interestingly, we observed a recovery in terms of discomfort, phantogeusia/parageusia and general taste alterations at the VI month observational time point, while a significant difference could still be observed at a later stage in terms of decline in basic taste, particularly hypo-ageusia (Table 3 and Fig. 1).
Chencharick et al. [18] in a longitudinal observational study suggested that the tumor bulk may be responsible for symptoms prior to treatment and that delivery of RT may lead to symptom exacerbation at a later stage. In this study, 74 HNC patients underwent exclusive RT with radical intent. Using a subjective questionnaire evaluating taste, a sixfold increase in dysgeusia by the 5th week of treatment was reported. [18].
In our study, we scored, on a weekly basis as for CTCAE 4.0 criteria, the following toxicity endpoints (Table 4): nausea, vomiting, mucositis, dysphagia, xerostomia, cutaneous toxicity, and dysgeusia. Grade 2 dysgeusia appeared to be the most common toxicity as assessed by physician along the whole treatment and up to the I month observation time point, while, at VI month from the end of RT, it was grade 2 xerostomia (23%).
De Graeff et al. [19], reviewing 107 HNC patients treated with surgery ± RT, found out that, at 1 year, taste was rated among the significantly worse QoL issues. Moreover, this study highlighted how dysgeusia tends to be significantly worse at 36 months when compared to baseline. In contrast with this conclusion, a study carried out by Rampling et al. [20], highlighted a 1-year post RT recovery of taste. An exhaustive counseling is crucial in this setting, especially when CMT is going to be offered. A study by Mowry et al. [21] showed how patients undergoing CMT for laryngeal and oropharyngeal cancers have similar perceptions of their QoL, except for saliva production, which was worse for oropharyngeal. The difficulty to swallow and chew, and the taste alteration were similar among the two groups. Overall, a good QoL was reported after treatment [12]. The RT technique can consistently impact on QoL. In a NPC (nasopharingeal cancer) patient population analysis by Fang et al., the cohort treated with IMRT had both statistically and clinically significant improvement in global QoL, fatigue, taste/smell, dry mouth, and feeling ill at the time point of 3 months after RT, compared to 3D conformal radiotherapy [22].
Another endopoint of investigation was nausea trend during treatment and follow-up. Radiation-induced nausea is extremely frequent [9, 10]. Factors increasing the risk of nausea onset during treatment include: radiation dose to the dorsal vagal complex of the brainstem (including the area postrema), low neck field, young age [13]. Thus, patient characteristics and tumor site have an impact on the risk of nausea. Together, nausea and dysgeusia, can increase the risk of treatment compliance impairment. While the enhanced negative impact on patient’s compliance and QoL can easily be related to the presence of both toxicities, the mutual correlation between them has yet to be fully clarified. We tried to prevent and manage nausea and dysgeusia in our patient cohort, using a ginger-based supplement. Ginger (Zingiber officinale) is a spice traditionally used to treat nausea and vomiting, given its capacity to accelerate gastric emptying and stimulate gastric antral contractions. This effect is due to the presence of gingerols and shogaols and their activities on cholinergic M receptors and serotonergic 5-HT receptors [12]. With the positive impact on preventing nausea, ginger can limit at the same time the onset of dysgeusia. Despite the widespread use of antiemetics, nausea continues to be a frequent toxicity among oncological patients [9, 23]. Ginger can play a role in its management, alleviating symptoms through a combination of anti-inflammatory and anti-spasmodic activities. Its use has been investigated among oncological patients, especially in terms of CT-induced nausea (CIN). A large randomized, double-blind, multicenter trial showed that doses in the range of 0.5–1.0 g on a daily basis, significantly reduced acute CIN in patients receiving standard antiemetics [23]. On the other hand, researchers also showed that ginger might be an effective adjuvant treatment for chemotherapy-induced nausea and vomiting but recommendations for the its use in this oncological setting are still premature [24].
In our cohort, no patient was symptomatic at baseline as for nausea, and G2 events (according to CTCAE 4.0) were observed in 13% of patients at III week, with an optimal recovery with or without antiemetic drugs. We suggest that the limited percentage of patients experiencing ≥ G2 nausea can be related to the prophylactic use of ginger-based supplements, even if a control arm is lacking in the present study. Noteworthly, no patient experienced G3 nausea. We also aimed to clarify the relative influence between dysgeusia and nausea. Interestingly, a significant correlation between nausea and the dysgeusia dimension of diet-related issues, namely discomfort, was observed (details in Table 5). From II to VI, we highlighted a statistically significant correlation between nausea and discomfort (p < 0.05). Interestingly, a statistically significant relationship between nausea and the onset of dysgeusia, in dimensional terms of decline in basic taste (II week) and phantogeusia/parageusia (III week) was observed as well.
Conclusions
Dysgeusia and nausea, as a consequence of HNC therapy, are significant clinical endpoints to be fully and prospectively investigated [17]. Our prospective clinical data point out the multidimensional pattern of dysgeusia and its trend during RT or CMT in HN cancer patients. We observed a similar trend for both quantitative and qualitative alterations during treatment and early follow-up. At a later stage, significant impairment is still present for decline in basic taste, particularly hypo-ageusia. Even with the limitations of our study (slender sample size, short-term follow-up, the absence of a control arm), nausea, and discomfort seem to have a significant mutual correlation. The prophylactic use of ginger may to have limited the number of patients experiencing clinically significant nausea and, supposedly, reduced taste impairment. Our clinical data are potentially useful for future comparison.
References
Franco P, Potenza I, Schena M, Riva G, Pecorari G, Demo PG, et al. Induction chemotherapy and sequential concomitant chemo-radiation in locally advanced head and neck cancers: how induction-phase intensity and treatment breaks may impact on clinical outcomes. Anticancer Res. 2015;35:6247–54.
Franco P, Fiorentino A, Dionisi F, et al. Combined modality therapy for thoracic and head and neck cancer: a review of updated literature based on a consensus meeting. Tumori. 2016;102:459–71.
Franco P, Potenza I, Moretto F, Segantin M, Grosso M, Lombardo A, et al. Hypericum perforatum and neem oil for the management of acute skin toxicity in head and neck cancer patients undergoing radiation of chemo-radiation: a single-arm prospective observational study. Radiat Oncol. 2014;29:297.
Franco P, Martini S, Di Muzio J, Cavallin C, Arcadipane F, Rampino M, et al. Prospective assessment of oral mucositis and its impact on quality of life and patient-reported outcomes during radiotherapy for head and neck. Med Oncol. 2017;34:81.
Hovan AJ, Williams PM, Stevenson-Moore P, Wahlin YB, Ohrn KEO, Elting LS, et al. A systematic review of dysgeusia induced by cancer therapies. Support Care Cancer. 2010;18:1081–7.
Kano T, Kanda K. Development and validation of a chemotherapy-induced taste alteration scale. Oncol Nurs Forum. 2013;40:79–85.
Campagna S, Gonnella S, Stuardi M, Sperlinga R, Cerponi M, Olivero M, et al. Italian validation of the chemotherapy induced taste alteration scale. Assist Inferm Ric. 2016;35:22–8.
Sozeri E, Kutluturkan S. The validity and reliability of turkish version of the chemotherapy-induced taste alteration scale (CiTAS). Clin Nurs Res. 2016;2:235–49.
McKenzie E, Zaki P, Raman S, Olson R, McFarlane T, DeAngelis C, et al. Radiation-induced nausea and vomiting: a comparison between MASCC/ESMO, ASCO, and NCCN antiemetic guidelines. Support Care Cancer. 2019;27:783–91.
Dennis K, Zhang L, Lutz S, van Baardwijk A, van der Linden Y, Holt T, et al. International patterns of practice in the management of radiation therapy-induced nausea and vomiting. Int J Radiat Oncol Biol Phys. 2012;84:49–60.
Maranzano E, De Angelis V, Pergolizzi S, Lupattelli M, Frata P, Spagnesi S, et al. A prospective observational trial on emesis in radiotherapy: analysis of 1020 patients recruited in 45 Italian radiation oncology centres. Radiother Oncol. 2010;94:36–41.
Giacosa A, Morazzoni P, Bombardelli E, Riva A, Bianchi Porro G, Rondanelli M. Can nausea and vomiting be treated with ginger extract? Eur Rev Med Pharmacol Sci. 2015;19:1291–6.
Monroe AT, Reddy SC, Gibbs GL, White GA, Peddada AV. Factors associated with radiation-induced nausea and vomiting in head and neck cancer patients treated with intensity modulated radiation therapy. Radiother Oncol. 2008;87:188–94.
Heckmann SM, Hujoel P, Habiger S, Friess W, Wichmann M, Heckmann JG, et al. Zinc gluconate in the treatment of dysgeusia—a randomized clinical trial. J Dent Res. 2005;84:35–8.
Halyard MY. Taste and smell alterations in cancer patients—real problems with few solutions. J Support Oncol. 2009;7:68–9.
Steinback S, Hummel T, Böhner C, Berktold S, Hundt W, Kriner M, et al. Qualitative and quantitative assessment of taste and smell changes in patients undergoing chemotherapy for breast cancer or gynecologic malignancies. J Clin Oncol. 2009;27:1899–905.
Irune E, Dwivedi RC, Nutting CM, Harrington KJ. Treatment-related dysgeusia in head and neck cancer patients. Cancer Treat Rev. 2014;40:1106–17.
Chencharick JD, Mossman KL. Nutritional consequences of the radiotherapy of head and neck cancer. Cancer. 1983;51:811–5.
de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110:98–106.
Rampling T, King H, Mais KL, Humphris GM, Swindell R, Sykes A, et al. Quality of life measurement in the head and neck cancer radiotherapy clinic: is it feasible and worthwhile? Clin Oncol. 2003;15:205–10.
Mowry SE, LoTempio MM, Sadeghi A, Wang KH, Wang MB. Quality of life outcomes in laryngeal and oropharyngeal cancer patients after chemoradiation. Otolaryngol Head Neck Surg. 2006;135:565–70.
Fang FM, Chien CY, Tsai WL, Chen HC, Hsu HC, Lui CC, et al. Quality of life and survival outcome for patients with nasopharyngeal carcinoma receiving three-dimensional conformal radiotherapy vs. intensity-modulated radiotherapy: a longitudinal study. Int J Radiat Oncol Biol Phys. 2008;72:356–64.
Ryan JL, Heckler CE, Roscoe JA, Dakhil SR, Kirshner J, Flynn PJ, et al. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: a URCC CCOP study of 576 patients. Support Care Cancer. 2012;20:1479–89.
Marx W, McCarthy AL, Ried K, Vitetta L, McKavanagh D, Thomson D, et al. Can ginger ameliorate chemotherapy-induced nausea? Protocol of a randomized double blind, placebo-controlled trial. BMC Complement Altern Med. 2014;9:14–134.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they do not have any conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The present study has been reviewed and approved by the Internal Review Board of the Department of Oncology of the University of Turin at AOU Citta’ della Salute e della Scienza, Turin, Italy.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martini, S., Iorio, G.C., Arcadipane, F. et al. Prospective assessment of taste impairment and nausea during radiotherapy for head and neck cancer. Med Oncol 36, 44 (2019). https://doi.org/10.1007/s12032-019-1269-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-019-1269-x