Abstract
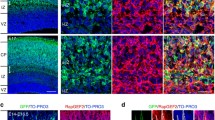
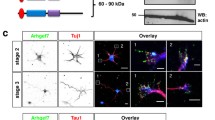
Rap1 and N-cadherin regulate glia-independent translocation of cortical neurons. It remains unclear how Rap1 regulates N-cadherin-mediated neuronal migration. Here, we overexpressed Rap1gap in mouse brains (embryonic day 16) to inactivate Rap1, and observed that neurons did not migrate to the outer layer. We confirmed that Rap1 was involved in the regulation of late neurons in vivo. Rap1gap overexpression and Rap1 suppression in CHO cells decreased the expression of cytoskeletal proteins such as tubulin. Changes in the expression of cell morphology regulators, such as N-cadherin and β-catenin, were also observed. Inhibition of N-cadherin in mouse brains prevented neuronal migration to the outer layer. The morphology of CHO cells was changed after overexpression of Rap1gap. We propose that Rap1 regulates the expression of N-cadherin during embryonic development, which affects β-catenin expression. Beta-catenin in turn regulates cytoskeletal protein expression, ultimately affecting neuronal morphology and migration.







Similar content being viewed by others
References
Aberle H, Schwartz H, Kemle R (1996) Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 61(4):514–523
Campbell K, Gotz M (2002) Radial glia: multi-purpose cells for vertebrate brain development. Trends Neurosci 25(5):235–238
Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U (2011) Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron 69(3):482–497
Gleeson JG, Walsh CA (2000) Neuronal migration disorders: from genetic diseases to developmental mechanisms. Trends Neurosci 23(8):352–359
Goto M, Mitra RS, Liu M, Lee J, Henson BS, Carey T, Bradford C, Prince M, Wang CY, Fearon ER, D’Silva NJ (2010) Rap1 stabilizes beta-catenin and enhances beta-catenin-dependent transcription and invasion in squamous cell carcinoma of the head and neck. Clin Cancer Res 16(1):65–76
Gumbiner BM (1996) Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84(3):345–357
Hajra KM, Fearon ER (2002) Cadherin and catenin alterations in human cancer. Genes Chromosom Cancer 34(3):255–268
Hisata S, Sakisaka T, Baba T, Yamada T, Aoki K, Matsuda M, Takai Y (2007) Rap1-PDZ-GEF1 interacts with a neurotrophin receptor at late endosomes, leading to sustained activation of Rap1 and ERK and neurite outgrowth. J Cell Biol 178(5):843–860
Jossin Y (2011) Polarization of migrating cortical neurons by Rap1 and N-cadherin: revisiting the model for the Reelin signaling pathway. Small GTPases 2(6):322–328
Jossin Y, Cooper JA (2011) Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci 14(6):697–703
Kawauchi T, Chihama K, Nabeshima Y, Hoshino M (2006) Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol 8(1):17–26
Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M (1989) A ras-related gene with transformation suppressor activity. Cell 56(1):77–84
Lee GH, D’Arcangelo G (2016) New insights into reelin-mediated signaling pathways. Front Cell Neurosci 10:122
Luskin MB, Shatz CJ (1995) Neurogenesis of the cat's primary visual cortex. J Comp Neurol 242(4):611–631
Nadarajah B, Parnavelas JG (2002) Modes of neuronal migration in the developing cerebral cortex. Nat Rev Neurosci 3(6):423–432
Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL (2001) Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci 4(2):143–150
Pearlman AL, Faust PL, Hatten ME, Brunstrom JE (1998) New directions for neuronal migration. Curr Opin Neurobiol 8(1):45–54
Rakic P (1972) Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol 145(1):61–83
Rashid T, Banerjee M, Nikolic M (2001) Phosphorylation of Pak1 by the p35/Cdk5 kinase affects neuronal morphology. J Biol Chem 276(52):49043–49052
Samuels BA, Tsai LH (2004) Nucleokinesis illuminated. Nat Neurosci 7(11):1169–1170
Santana J, Marzolo MP (2017) The functions of Reelin in membrane trafficking and cytoskeletal dynamics: implications for neuronal migration, polarization and differentiation. Biochem J 474(18):3137–3165
Shah S, Brock EJ, Jackson RM, Ji K, Boerner JL, Sloane BF, Mattingly RR (2018) Downregulation of Rap1Gap: a switch from DCIS to invasive breast carcinoma via ERK/MAPK activation. Neoplasia 20(9):951–963
Walsh CA, Goffinet AM (2000) Potential mechanisms of mutations that affect neuronal migration in man and mouse. Curr Opin Genet Dev 10(3):270–274
Wang S, Brunne B, Zhao S, Chai X, Li J, Lau J, Failla AV, Zobiak B, Sibbe M, Westbrook GL, Lutz D, Frotscher M (2018) Trajectory analysis unveils Reelin’s role in the directed migration of granule cells in the dentate gyrus. J Neurosci 38(1):137–148
Ye T, Ip JP, Fu AK, Ip NY (2014) Cdk5-mediated phosphorylation of RapGEF2 controls neuronal migration in the developing cerebral cortex. Nat Commun 5(4826):4826
Funding
This work was supported by a grant from Henan Province Natural Science Foundation (162300410214, 14B310007), the Henan Province University youth researcher support program project (2015GGJS-133), the National Science Foundation of China (No 81771226, 81600987), the support project for the Disciplinary group of Psychology and Neuroscience, Xinxiang Medical University (2016PN-KFKT-03, 20172DCG-03), the Science and Technology Innovation Talents Support Program of Henan Universities and Xinxiang City (14HASTIT032, CXRC16003), Xinxiang major science and technology projects (ZD17008), the PhD Research Startup Foundation (505090) of Xinxiang Medical University, and Innovation of College Students Project (SKYLS005, 201710472011).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Juntang Lin and Ciqing Yang. Performed the experiments: Ciqing Yang, Xiaoying Li, Bichao Zhang, Sulei Fu, Shuanqing Li, Jianing Shen, Lihong Guan, and Liang Qiao. Analyzed the data: Ciqing Yang. Wrote the paper: Ciqing Yang.
Corresponding author
Ethics declarations
Experiments were approved by the Animal Care Committee of Xinxiang Medical University (No. 030032). All animal protocols were conducted under the guidelines of The Ministry of Science and Technology of the People’s Republic of China [(2006)398].
Competing Interests
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, C., Li, X., Zhang, B. et al. The Mechanism of Rap1 Regulates N-cadherin to Control Neuronal Migration. J Mol Neurosci 68, 539–548 (2019). https://doi.org/10.1007/s12031-019-01316-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-019-01316-w