Abstract
Purpose
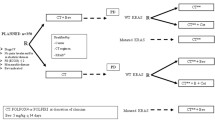
Bevacizumab is a standard first-line (L1) treatment for metastatic colorectal cancer (mCRC) patients regardless of RAS status. This retrospective study examined treatment patterns and outcomes in a community oncology sample of KRAS mutant mCRC patients treated with chemotherapy (C) or C plus bevacizumab (CB) in L1.
Methods
This study used medical records from the Vector Oncology Data Warehouse. Eligible patients were confirmed KRAS mutant mCRC and received L1 C or CB. Kaplan-Meier analysis assessed L1 progression-free survival (PFS) and overall survival (OS). Cox regression models examined the interaction of tumor location (R/L) with treatment.
Results
CB (n = 264) compared to C (n = 109) patients were younger, less likely performance status (PS) impaired, and more likely with liver metastases. Median unadjusted PFS was 10.41 months (95% CI 9.0–11.3) in CB and 7.66 months (95% CI 6.5–9.1) in C patients (p = 0.174). Median unadjusted OS was 26.91 months (95% CI 24.3–29.3) in CB and 23.33 months (95% CI 19.7–29.2) in C patients (p = 0.571). For patients with right- vs. left-sided tumors, C (but not CB)-treated patients had higher adjusted risk for progression (HR = 1.715, 95% CI 1.108, 2.653; p = 0.015).
Conclusions
CB- vs. C-treated KRAS mutant mCRC patients may have a meaningful PFS benefit. Patients with right-sided tumors treated with C were at higher risk for disease progression than patients with left-sided tumors. Tumor location had no significant effect on outcomes in the CB cohort.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017; https://doi.org/10.3322/caac.21387.
Hu CY, Bailey CE, You YN, Skibber JM, Rodriguez-Bigas MA, Feig BW, et al. Time trend analysis of primary tumor resection for stage IV colorectal cancer: less surgery, improved survival. JAMA Surg. 2015;150(3):245–51. https://doi.org/10.1001/jamasurg.2014.2253.
de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–47.
Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22(1):23–30. https://doi.org/10.1200/JCO.2004.09.046.
Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25(13):1670–6. https://doi.org/10.1200/JCO.2006.09.0928.
Starling N, Tilden D, White J, Cunningham D. Cost-effectiveness analysis of cetuximab/irinotecan vs active/best supportive care for the treatment of metastatic colorectal cancer patients who have failed previous chemotherapy treatment. Br J Cancer. 2007;96(2):206–12.
Capdevila J, Ramos FJ, Macarulla T, Elez E, Tabernero J. The role of salvage treatment in advanced colorectal cancer. Crit Rev Oncol Hematol. 2009;71(1):53–61.
Kelly H, Goldberg RM. Systemic therapy for metastatic colorectal cancer: current options, current evidence. J Clin Oncol. 2005;23(20):4553–60. https://doi.org/10.1200/JCO.2005.17.749.
Tabernero J, van Cutsem E, Lakomy R, Prausova J, Ruff P, van Hazel G, et al. Results from VELOUR, a phase III study of aflibercept versus placebo in combination with FOLFIRI for the treatment of patients with previously treated metastatic colorectal cancer Abstract 6LBA. Paper presented at the ECCO-ESMO 2011, Stockholm Sweeden,
Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. https://doi.org/10.1016/S0140-6736(12)61900-X.
Zaniboni A. New active drugs for the treatment of advanced colorectal cancer. World J Gastrointest Surg. 2015;7(12):356–9. https://doi.org/10.4240/wjgs.v7.i12.356.
Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29–37. https://doi.org/10.1016/S1470-2045(12)70477-1.
Kubicka S, Greil R, Andre T, Bennouna J, Sastre J, Van Cutsem E, et al. Bevacizumab plus chemotherapy continued beyond first progression in patients with metastatic colorectal cancer previously treated with bevacizumab plus chemotherapy: ML18147 study KRAS subgroup findings. Ann Oncol. 2013;24(9):2342–9. https://doi.org/10.1093/annonc/mdt231.
The National Comprehensive Cancer Network (NCCN) (2016) Clinical Practice Guidelines in Oncology: Colon Cancer, Version 2.2016. The National Comprehensive Cancer Network. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Accessed 22 Feb 2016.
Amgen (2015) Drug Label for Panitumumab. Amgen, Inc. http://pi.amgen.com/united_states/vectibix/vectibix_pi.pdf. Accessed 11 May 2015.
Bristol-Myers Squibb and Lilly (2015) Cetuximab Drug Label. Bristol-Myers Squibb and Lilly. http://packageinserts.bms.com/pi/pi_erbitux.pdf. Accessed 11 May 2015.
Venook AP, Niedzwiecki D, Innocenti F, Fruth B, Greene C, O’Neil BH, et al. (2016) Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). ASCO Meeting Abstracts 34 (15_suppl):3504.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. https://doi.org/10.1016/S0140-6736(10)61381-5.
Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26(12):2013–9. https://doi.org/10.1200/JCO.2007.14.9930.
Bencsikova B, Bortlicek Z, Halamkova J, Ostrizkova L, Kiss I, Melichar B, et al. Efficacy of bevacizumab and chemotherapy in the first-line treatment of metastatic colorectal cancer: broadening KRAS-focused clinical view. BMC Gastroenterol. 2015;15:37. https://doi.org/10.1186/s12876-015-0266-6.
von Einem JC, Heinemann V, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, et al. Left-sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild-type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK-0104 trial. J Cancer Res Clin Oncol. 2014;140(9):1607–14. https://doi.org/10.1007/s00432-014-1678-3.
Funding
This work was funded by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
SO and NS are employed by and own stock in Genentech, Inc. SSH and MW are consultants for Genentech. JH is on an advisory board for Genentech, with her institution receiving her honorarium. In addition, JH’s institution has received research support from Genentech. YZ has received travel support from Genentech. AH reported no conflicts of interest. Genentech sponsored this study and provided financial support for the conduct of the research and for preparation of the article. Genentech collaborated on the design of the study, interpretation of the analyses, and in the decision to submit the article for publication, but did not have a direct role in data collection, data analysis, or writing of the report.
Research Involving Human Participants
This research was reviewed and approved by IntegReview Institutional Review Board, in Austin, Texas. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
For this type of study, formal consent is not required.
Availability of Data and Material
Vector Oncology does not make datasets publicly available because study data are used under license from source practices. Vector Oncology will consider requests to access study datasets on a case-by-case basis.
Rights and permissions
About this article
Cite this article
Houts, A.C., Ogale, S., Zafar, Y. et al. Progression-Free Survival in Patients Receiving Chemotherapy Alone (C) or Chemotherapy with Bevacizumab (CB) for First-Line Treatment of KRAS Mutant Metastatic Colorectal Cancer in Community Oncology Settings. J Gastrointest Canc 50, 16–22 (2019). https://doi.org/10.1007/s12029-017-0017-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-017-0017-8