Abstract
Background
Rebleeding from a ruptured aneurysm increases the risk of unfavorable outcomes after subarachnoid hemorrhage (SAH) and is prevented by early aneurysm occlusion. The role of antifibrinolytics before aneurysm obliteration remains controversial. We investigated the effects of tranexamic acid on long-term functional outcomes of patients with aneurysmal SAH (aSAH).
Methods
This was a single-center, prospective, observational study conducted in a high-volume tertiary hospital in a middle-income country from December 2016 to February 2020. We included all consecutive patients with aSAH who either received or did not receive tranexamic acid (TXA) treatment. Multivariate logistic regression analysis using propensity score was used to evaluate the association of TXA use with long-term functional outcomes, measured by the modified Rankin Scale (mRS) at 6 months.
Results
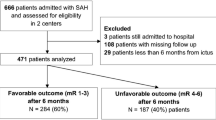
A total of 230 patients with aSAH were analyzed. The median (interquartile range) age was 55 (46–63) years, 72% were women, 75% presented with good clinical grade (World Federation of Neurological Surgeons grade 1–3), and 83% had a Fisher scale of 3 or 4. Around 80% of patients were admitted up to 72 h from ictus. The aneurysm occlusion method was surgical clipping in 80% of the patients. A total of 129 patients (56%) received TXA. In multivariable logistic regression using inverse probability treatment weighting, the long-term rate of unfavorable outcomes (modified Rankin scale 4–6) was the same in the TXA and non-TXA groups (61 [48%] in TXA group vs. 33 [33%] in non-TXA group; odds ratio [OR] 1.39, 95% confidence interval [CI] 0.67–2.92; p = 0.377). The TXA group had higher in-hospital mortality (33 vs. 11% in non-TXA group; OR 4.13, 95% CI 1.55–12.53, p = 0.007). There were no differences between the groups concerning intensive care unit length of stay (16 ± 11.22 days in TXA group vs. 14 ± 9.24 days in non-TXA group; p = 0.2) or hospital (23 ± 13.35 days in TXA group vs. 22 ± 13.36 days in non-TXA group; p = 0.9). There was no difference in the rates of rebleeding (7.8% in TXA group vs. 8.9% in non-TXA group; p = 0.31) or delayed cerebral ischemia (27% in TXA group vs. 19% in non-TXA group; p = 0.14). For the propensity-matched analysis, 128 individuals were selected (64 in TXA group and 64 in non-TXA group), and the rates of unfavorable outcomes at 6 months were also similar between groups (45% in TXA group and 36% in non-TXA group; OR 1.22, 95% CI 0.51–2.89; p = 0.655).
Conclusions
Our findings in a cohort with delayed aneurysm treatment reinforce previous data that TXA use before aneurysm occlusion does not improve functional outcomes in aSAH.


Similar content being viewed by others
Change history
11 October 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12028-023-01866-3
References
Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50(5):1413–8.
Maher M, Schweizer TA, Macdonald RL. Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke. 2020;51(4):1326–32.
Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen MDI, Rinkel GJE, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. 2019;76(5):588–97.
Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. 2020;54(2):171–9.
Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820.
Germans MR, Coert BA, Vandertop WP, Verbaan D. Time intervals from subarachnoid hemorrhage to rebleed. J Neurol. 2014;261(7):1425–31.
Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the stroke council. Am Heart Assoc Stroke. 2009;40(3):994–1025.
Diringer MN, Bleck TP, Claude Hemphill J, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society’s multidisciplinary consensus conference. Neurocrit Care. 2011;15(2):211–40.
Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2012;43(6):1711–37.
Post R, Germans MR, Boogaarts HD, Ferreira Dias Xavier B, Van den Berg R, Coert BA, et al. Short-term tranexamic acid treatment reduces in-hospital mortality in aneurysmal sub-arachnoid hemorrhage: a multicenter comparison study. PLoS One. 2019;14(2):e0211868.
Post R, Germans MR, Tjerkstra MA, Vergouwen MDI, Jellema K, Koot RW, et al. Ultra-early tranexamic acid after subarachnoid haemorrhage (ULTRA): a randomised controlled trial. Lancet. 2021;397(10269):112–8.
Baharoglu MI, Germans MR, Rinkel GJ, Algra A, Vermeulen M, van Gijn J, et al. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Datab Syst Rev. 2013. https://doi.org/10.1002/14651858.CD001245.pub2(8):CD001245.
Ren J, Qian D, Wu J, Ni L, Qian W, Zhao G, et al. Safety and efficacy of tranexamic acid in aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. Front Neurol. 2021;12:710495.
Shi M, Yang C, Chen ZH, Xiao LF, Zhao WY. Efficacy and safety of tranexamic acid in aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis of randomized controlled trials. Front Surg. 2021;8:790149.
Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988; 68(6):985–986.
Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006;59(1):21–7.
Baggio JA, Santos-Pontelli TE, Cougo-Pinto PT, Camilo M, Silva NF, Antunes P, et al. Validation of a structured interview for telephone assessment of the modified Rankin Scale in Brazilian stroke patients. Cerebrovasc Dis. 2014;38(4):297–301.
Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–70.
Greifer N. Weighting for covariate balance in observational studies. 2020.
Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–7.
Li X, Shen C. Doubly robust estimation of causal effect: upping the odds of getting the right answers. Circ Cardiovasc Qual Outcomes. 2020;13(1):e006065.
Funding
The authors declare no funding was received.
Author information
Authors and Affiliations
Contributions
CBR, VH, PHRS, MLT, LP, FKG, RT, BG, CR, and PK participated in data collection and adjudication of clinical data. CBR, AAR, RT, BG, CR, FAB, and PK contributed to the study conception and design, and data interpretation. CBR, BG, LSLB, and PK performed data processing, statistical analysis, and statistical revision. CBR, AAR, BG, and PK drafted the first version of the article. CBR, BG, CR, FAB, and PK supervised the study. All authors had full access to data, participated in data interpretation, revised the article, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
All authors declared that they have no conflict of interest.
Ethical Approval/Informed Consent
We confirm adherence to ethical guidelines and indicate ethical approvals in the text. Research Ethics Committee (CAAE: 27362719.6.0000.5530).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original article has been updated to correct the name of coauthor from Leticia Peterson to Letícia Petterson.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rynkowski, C.B., Hegele, V., Soares, P.H.R. et al. Effects of Tranexamic Acid in Patients with Subarachnoid Hemorrhage in Brazil: A Prospective Observational Study with Propensity Score Analysis. Neurocrit Care 39, 191–197 (2023). https://doi.org/10.1007/s12028-023-01732-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-023-01732-2