Abstract
Background
Clinical approach to ventilator-associated pneumonia (VAP) in the neurocritical care unit (NCCU) varies widely among physicians despite training and validated criteria.
Methods
Prospective observational study of all mechanically ventilated patients with suspected VAP over 18 months in an academic NCCU. Patients meeting VAP criteria by a surveillance program (SurvVAP) were compared to treated patients who did not meet surveillance criteria (ClinVAPonly). We identified appropriate/potentially inappropriate antibiotic treatment and factors associated with excessive antibiotic days (EAD).
Results
Of 622 ventilated patients, 83 cases were treated as VAP. Of these, 26 (31.3 %) had VAP by CDC criteria (SurvVAP) (VAP rate = 7.3 cases/1,000 ventilator days). Clinical features significantly more prevalent in SurvVAP cases (vs. ClinVAPonly) were change in sputum character, tachypnea, oxygen desaturation, persistent infiltrate on chest X-ray and higher clinical pulmonary infection score, but not positive sputum culture. Treatment with pneumonia-targeted antibiotics for >8 days was significantly more common in ClinVAPonly versus SurvVAP patients (73.7 vs. 30.8 %, p < 0.001) even after excluding patients with other infections (p = 0.001). Based on current guidelines, the ClinVAPonly group contributed 225 EAD, including 38 vancomycin days, 70 piperacillin–tazobactam days and 85 cephalosporin days with cost figure over four times that of EAD in SurvVAP group. No pre-specified factors were associated with continued VAP treatment beyond 8 days.
Conclusions
Incongruency between clinically and surveillance-defined VAP is common in acute neurological disease although outcomes did not differ between groups. Clinician behaviors rather than clinical factors may contribute to prolonged prescribing.
Similar content being viewed by others
Introduction
In mechanically ventilated patients, ventilator-associated pneumonia (VAP) is the most common nosocomial infection accounting for approximately 50 % of all antibiotics given in the intensive care unit (ICU) [1, 2]. VAP is also associated with increased duration of mechanical ventilation, length of ICU and hospital stay and increased healthcare costs [3–5]. Moreover, VAP is now on the list of hospital-acquired infections qualifying for non-payment by Medicare. In 2012, among National Healthcare Safety Network (NHSN) participating facilities the incidence of VAP in neurocritical care units (NCCU) ranged from 2.1 (neurosurgical units) to 3.0 (neurologic units) per 1,000 ventilator days [6].
Diagnosis of VAP remains a challenge due to the absence of a universally accepted definition [7, 8]. In the neurocritical care population, VAP diagnosis is particularly problematic because clinical findings such as fever, leukocytosis and altered mental status (all components of VAP definitions [9]) are ubiquitous in acute neurologic disease irrespective of infection. The initiation of a VAP surveillance program in our NCCU revealed significant discrepancy between numbers of surveillance-defined and treated cases suggesting that over-diagnosis and potential inappropriate use of antibiotic therapy may be occurring. Data examining the excessive use of antibiotics for VAP in patients without VAP are limited and have not been investigated in neurocritical care patients. Swoboda et al. [10] reported that half of empiric antibiotic use for VAP in two surgical ICUs was prescribed for patients without pneumonia. Our group previously found that in six multidisciplinary ICUs, inappropriate diagnosis and treatment of VAP was common although clinical differences between patients without VAP who had antibiotics continued or discontinued beyond 3 days were minimal, suggesting that clinician behaviors contribute to unnecessary prescribing [11]. Factors driving diagnostic and treatment decisions were not examined by ICU type. The objectives of this study were the following: (1) to examine neurocritical care patients diagnosed and treated for VAP who did not meet Center for Disease Control and Prevention (CDC)/NHSN surveillance definitions to compare clinical differences with patients treated for VAP who did meet these criteria within the same time period; (2) to quantify appropriate and potentially inappropriate antibiotic use for VAP in both clinical VAP only (not meeting CDC criteria) and surveillance-defined VAP cases; and (3) to identify factors associated with continuation of antibiotics beyond 8 days in patients with clinical VAP only.
Methods
Study Design and Setting
This was a prospective study of consecutive intubated patients admitted to a NCCU in a university hospital over 18 months from January 1, 2011, to June 30, 2012. The NCCU has 24 beds and provides complete ICU management for a mixed neurosurgical/neurologic patient population that includes perioperative brain and spine patients, neurotrauma, strokes, brain hemorrhages, and a wide array of neurologic illnesses with critical care needs. This study was approved by the hospital’s institutional review board committee.
Patient Selection
We included all adult patients who were treated for VAP while mechanically ventilated as well as within 24 h of liberation from mechanical ventilation. Patients who died during VAP treatment were included. Patients were excluded if ventilated for <48 h or if treated for documented tracheitis (without pneumonia). There were no recurrent episodes of VAP identified. Figure 1 shows identification of patient groups.
Diagnosis of Pneumonia
Surveillance Definition of Pneumonia
All adult (age ≥18 years) mechanically ventilated patients in the NCCU were prospectively identified by a dedicated hospital epidemiology and infection control (HEIC) practitioner (ICP) who performed standardized daily screening using the following criteria: radiographic appearance of a new or progressive pulmonary infiltrate, and two of the three following: (1) fever, (2) leukocytosis and (3) purulent tracheobronchial secretions. The ICP and a neurointensivist (W.Z.) reviewed each potential case of VAP, and consensus was achieved as to whether the case met CDC/NHSN criteria for VAP, either pneumonia I (PNU1) or PNU2 [12]. For PNU1, specific criteria required patients to fulfill one radiographic, one systemic, and two pulmonary criteria: (1) radiographic criteria: two or more serial chest radiographs with at least one of the following: (a) a new or progressive and persistent infiltrate, (b) consolidation or (c) cavitation; systemic criteria: one of the three following findings: (1) fever (>38 °C or >100.4 °F) or body temperature either <36.0 °C with no other recognized cause, (2) leukopenia (<4,000 WBC/mm3) or leukocytosis (>12,000 WBC/mm3), and (3) for adults >70 years old, altered mental status with no other recognized cause; pulmonary criteria: at least two of the following: (i) new onset of purulent sputum or a change in sputum character or increased respiratory secretions or increased suctioning requirements, (ii) new onset or worsening cough, or dyspnea, or tachypnea, (iii) rales or bronchial breath sounds, and (iv) worsening gas exchange [e.g., O2 desaturations (e.g., PaO2/FiO2 ≤240), increased oxygen requirements or increased ventilator demand].
Bacterial or fungal pneumonia with specific laboratory findings (PNU2) was defined as the above, but requiring only one pulmonary criteria (i–iv), and at least one of the following: (a) positive growth in blood culture not related to another source of infection, (b) positive growth in culture of pleural fluid, (c) positive quantitative culture from minimally contaminated LRT specimen (e.g., BAL or protected specimen brushing), (d) 5 % BAL-obtained cells contain intracellular bacteria on direct microscopic exam (e.g., Gram stain) or (e) histopathologic exam shows at least one of the following evidences of pneumonia: (i) abscess formation or foci of consolidation with intense polymorphonuclear (PMN) accumulation in bronchioles and alveoli, (ii) positive quantitative culture of lung parenchyma, and (iii) evidence of lung parenchyma invasion by fungal hyphae or pseudohyphae.
Clinical Definition of Pneumonia
The clinical diagnosis of pneumonia was determined by the NCCU team according to normal clinical practice. To count as clinically treated pneumonia, we required evidence of antibiotic therapy targeted at VAP in the daily progress note. Quantitative diagnostic thresholds for bacterial cultures from BAL and miniBAL at our institution are 104 colony forming units/mL. Endotracheal aspirate sputum samples are not quantified. They are reported semiquantitatively only (light, moderate or heavy for first, second and third quadrant growth on a plate, respectively).
The NCCU team adheres to local hospital-wide antibiotic guidelines for VAP treatment. Practitioners were unaware of the ongoing study and had access to cumulative surveillance data, but not individual cases. No surveillance endotracheal cultures were performed.
Patients were divided into two groups. The surveillance VAP (SurvVAP) group met CDC criteria for PNU1 or PNU2. The clinical only VAP group (ClinVAPonly) included all patients who were treated for VAP but did not meet CDC/NHSN surveillance definition criteria.
All data were prospectively recorded. We recorded baseline demographic, medical history and radiologic data onto standardized forms. Number of days on the ventilator prior to initiation of antibiotics was recorded. The following variables were recorded at time of clinical diagnosis for all patients to determine whether they met CDC/NHSN criteria for VAP: new or progressive pulmonary infiltrate on chest X-ray, fever (>38 °C), leukopenia (<4,000 WBC/mm3), leukocytosis (>12,000 WBC/mm3), change in mental status [defined as decrease in Glasgow Coma Scale (GCS) by a score of one], new onset of purulent sputum (or a change in the character of sputum or increased respiratory secretions or increased suctioning requirements), new onset of worsening cough, dyspnea or tachypnea, rales or bronchial breath sounds, PaO2/FiO2 ≤240 and clinical pulmonary infection score (CPIS) [13]. VAP rate was defined as number of VAP cases divided by number of ventilator days × 1,000 (cases per 1,000 ventilator days).
Antimicrobial Management
For both groups, the date of initiation, class and duration of antibiotic(s) was reported. We collected all culture data (blood, sputum, urine, cerebrospinal fluid) from 24 h before and during “VAP” treatment until 48 h post-extubation. In patients who were treated with antibiotics for a coexisting infection, we recorded antibiotic type, start and duration of treatment to determine overlap of treatment. If a concomitant infection (culture positive or high clinical suspicion in the progress note) was being treated with antibiotics that overlapped with the treatment of VAP, only patients who received antibiotics for >72 h and in whom the clinical suspicion of VAP had developed during antimicrobial treatment were included. The following outcomes were collected: Antibiotic treatment targeted to VAP for >3 and >8 days, number of excess antibiotic days (EAD) and in-hospital mortality. Inappropriate antibiotic treatment was defined for the ClinVAPonly group as continuation of therapy beyond 72 h and for the SurvVAP group as >8 days of therapy for VAP unless the patient met the following criteria: (1) severe immunocompromised state, (2) VAP with associated bacteremia, (3) VAP due to MRSA, (4) initial antimicrobial treatment was not appropriate for the causative organisms or (5) infections caused by difficult-to-treat organisms (e.g., Pseudomonas aeruginosa or multidrug-resistant Acinetobacter baumanii), or with no improvement in clinical signs of infection.
EAD were defined as the cumulative number of days >3 of all antibiotics that were administered to the ClinVAPonly group toward VAP treatment, >8 days to the SurvVAP group and >14 days for VAP meeting criteria for longer duration (as indicated above) [14, 15]. EAD was calculated as the sum total of number of days of each antibiotic, when multiple antibiotics were administered. Antibiotic cost for EADs for each antibiotic group was calculated for each group using the acquisition cost of antibiotics per day from our hospital antibiotic guide.
Statistics
Demographic and clinical variables including VAP-associated clinical characteristics were compared between SurvVAP and ClinVAPonly groups and within the ClinVAPonly group between patients treated for >8 days of pneumonia-targeted antibiotics and those treated for ≤8 days. We did not analyze groups based on a 3-day treatment threshold due to small sample size in the shorter treatment group. Wilcoxon rank-sum test was used to compare variables with non-normal distributions. Student’s t test was used for continuous variables with normal distributions, and Chi-square or Fisher exact test was used for analysis of categorical data as appropriate. Statistical significance was assigned for p < 0.05. Data are presented as median ± interquartile range (iqr) or mean ± SD, unless otherwise indicated. Statistical analyses were performed using STATA 11.1 (STATA Corporation, College Station, TX, USA).
Results
A total of 622 mechanically ventilated patients were admitted to the NCCU over the 18-month period. Of these, 83 patients were treated for VAP of which 26 (31.3 %) fit CDC criteria for VAP (SurvVAP) and 57 (68.7 %) did not (ClinVAPonly). All patients identified in the SurvVAP group were treated for VAP. The VAP rate was 7.3 cases/1,000 ventilator days, and the ventilator utilization ratio was 0.30 (=ventilator days/patient days). In the SurVAP group, all 26 patients fit PNU1 criteria. Of all 83 patients treated for VAP, males comprised 51 (61.5 %) and mean age was 58.2 ± 1.83 (SEM) years. There was no significant difference in proportions of neurological (42.2 % of clinVAPonly group) and neurosurgical patients (57.8 %) in either group. With the exception of duration of antibiotic therapy >8 days, there were no significant differences in patient demographics, median ventilator and total antibiotic days and the presence of concomitant infection between the two study groups (Table 1).
The following VAP features were significantly more likely to be found in the SurvVAP group versus the ClinVAPonly group (Table 2): change in sputum character, tachypnea, oxygen desaturation, consolidation and persistent infiltrate on chest X-ray. Significance did not change after excluding patients with concomitant infection. Characteristics that were not different between groups were the presence of fever, leukocytosis/leukopenia, change in mental status, bronchial breath sounds, positive sputum culture and method by which sputum was obtained. Median CPIS was significantly lower in the ClinVAPonly group versus the SurvVAP group (3 vs. 5; p < 0.001). CPIS ≥6 at time of diagnosis occurred in 9/26 (35 %) of the SurvVAP group and 4/57 (7 %) of the ClinVAPonly group (p = 0.003).
A significantly greater proportion of ClinVAPonly patients received pneumonia-targeted antibiotics for >8 days (73.7 %) versus 30.8 % of SurvVAP patients (p < 0.001). This result did not change after excluding patients with concomitant infection (p = 0.001) or excluding patients with MDROs on sputum culture (p = 0.001). Comparing demographic and clinical VAP characteristics of patients in the ClinVAPonly group who were treated for >8 versus ≤8 days, the only factor which differed between groups was older age in the group treated for >8 days (60.9 ± 2.4 vs. 50.5 ± 4.4 years, respectively; p = 0.04).
Microbiology Results for ClinVAPonly and SurvVAP Groups
Figure 1 (Table 3) lists the organisms cultured from sputum in both groups. Sputum samples were obtained by endobronchial sampling, miniBAL or bronchoalveolar lavage (BAL). There were no fungal isolates. In the ClinVAPonly group, 45 % of cultures were polymicrobial (compared to 38 % in the SurvVAP group). A positive sputum culture for MDROs occurred in nine (15.8 %) patients in the ClinVAPonly group and three (11.5 %) in the SurvVAP group (p = 0.75). After excluding patients with MDROs, the same VAP features were significantly more likely to be found in the SurvVAP group versus the ClinVAPonly group (new change in sputum character, tachypnea, oxygen desaturation, consolidation and persistent infiltrate on chest X-ray). Within the ClinVAPonly group, the only significant difference between patients with MDROs and those without was the presence of a positive sputum culture (100 vs. 60.4 %; p = 0.02) (Fig. 2).
Antimicrobial Management
The median number of days from intubation to starting antibiotics was four in both groups (p = 0.53). Table 3 shows potential EADs administered to patients in both groups along with the estimated dollar value of treatment. By the most conservative estimate, excluding patients with concomitant infection and MDROs, treatment of SurvVAP for >8 days and ClinVAPonly (not meeting CDC definition for VAP) for >3 days resulted in 35 and 225 EADs, respectively. Comparing ClinVAPonly and SurvVAP groups without concomitant infection or MDRO’s, the cumulative cost for EADs amounted to $4,125.00 versus $940.00, a potential excess cost over four times in patients who did not meet the more stringent criteria for VAP (p = 0.0001).
In patients with a single bacterium isolated from sputum culture, the most common bacteria were MSSA (15 %), pseudomonas (14 %) and MRSA (11 %). Thirty-four patients had polymicrobial cultures (SurvVAP: 38 %, ClinVAPonly: 45 %); of these, 13 had mixed oral flora (seven in the ClinVAPonly group and six in the SurvVAP group). Five cultures grew normal flora (all in ClinVAPonly group), and two had no growth (all in ClinVAPonly group) (Table 4).
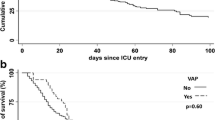
Tracheostomy rates, length of ICU and hospital stay and in-hospital mortality rates for the two groups did not differ.
Discussion
We report a high-risk population of neurocritical care patients in which clinical and surveillance diagnoses of VAP were often incongruent and patients with clinical only VAP (not meeting surveillance criteria) were significantly more likely to receive antibiotics for greater than the typical recommended 8-day course of treatment, even after excluding the presence of concomitant infections and MDROs. The potential for EAD was significant (225–300 EADs in 57 patients who were diagnosed as VAP but did not meet CDC surveillance criteria). Using the most conservative estimate, the antibiotic cost of EADs in the ClinVAPonly group was over four times that of the SurvVAP group with no significant difference in ventilator days, NCCU length of stay or in-hospital mortality between these groups.
This apparent variability between surveillance-defined and individual clinician-based diagnosis of VAP is consistent with prior studies which have reported inconsistencies in VAP diagnosis by trained reviewers in neurologic conditions [8, 16]. Kuusinen et al. [8] prospectively evaluated six different clinical criteria for diagnosing pneumonia in 390 neurosurgical patients and similar to our results found that physician diagnosis produced the highest pneumonia frequency, resulting in 34 false-positive cases compared to general surveillance methods (B) and 25 false-positive cases compared to consensus clinical criteria (C). The attending physicians also missed nine pneumonia cases according to the B criterion and 14 according to C. This study did not benefit from microbiological verification of pneumonia which may have led to over-diagnosis although microbiological findings appeared to be unnecessary for diagnosing VAP in the current study. Naidech et al. [16] demonstrated poor inter-rater reliability of incidence reporting of hospital-acquired pneumonia in neurocritical care patients. In their prospective study, 103 patients diagnosed with pneumonia by a neurointensivist were independently reviewed. The incidence of pneumonia ranged from 5 to 25 %, and inter-rater reliability was poor. Both studies concluded that methods for clinical diagnosis of VAP were unreliable.
The objective of this study was to determine specific components which differ between surveillance and clinically diagnosed VAP in patients with neurologic diseases. Compared to surveillance-defined VAP cases, patients treated clinically for VAP who did not meet surveillance definitions were significantly less likely to have a persistent infiltrate, change in sputum character, new onset cough/dyspnea/tachypnea or worsened gas exchange. Possible explanations for the high rate of VAP diagnosis by clinicians were (1) increased reliance on fever and leukocytosis which in the presence of nonpersistent or non-progressive changes in CXR and positive sputum culture may have decreased threshold for starting antibiotics; (2) lack of an alternative diagnosis or fear of assuming fever/leukocytosis was related to intracranial pathology; (3) reliance on fewer simultaneously present criteria compared to surveillance methods which require that many criteria be present simultaneously; and (4) a higher frequency of patients with change in mental status without recognized cause in the ClinVAPonly versus SurvVAP group (19.3 vs. 7.7 %) influencing antibiotic prescribing behavior in patients who just looked sicker.
While there is no data to suggest that physicians’ diagnoses are more or less accurate than criteria used for surveillance purposes [17], it has been reported that over half of patients diagnosed with VAP do not have the disease, while up to one-third of those with VAP are not diagnosed [18, 19]. In the general ICU population, delayed administration of appropriate antibiotics for VAP increases mortality [20, 21]. However, over-diagnosing VAP can lead to inappropriate antibiotic use and consequently to the emergence of multidrug-resistant organisms and invasive fungal infection in addition to increased cost and risk of adverse drug reactions [22, 23]. There is, therefore, a need to prioritize and combine clinical findings when considering the diagnosis. Klompas found that fever, leukocytosis or purulent secretions alone did not significantly change the probability of VAP defined by a histological gold standard, but that combining two of these with a new infiltrate increased likelihood of VAP [7]. The presence of a new infiltrate alone was only marginally useful which is consistent with an autopsy review of VAP which found that the only diagnostically useful radiographic sign was the presence of air bronchograms [24]. If we apply the ATS/IDSA criteria for starting empiric therapy to our population (the presence of a new or progressive radiographic infiltrate and at least two of three clinical features (fever >38 °C, leukocytosis or leukopenia and purulent secretions) [25], only 8/57 (14 %) from the ClinVAPonly group and 11/26 (42 %) from the SurvVAP group would have met ATS/IDSA criteria. This comparison suggests that other factors, especially reliance on endotracheal aspirates with non-quantitative culture likely enhanced false-positive VAP diagnosis in this study. The relatively high number of cultures in the ClinVAPonly group with polymicrobial, normal oral flora or negative cultures suggests that clinicians may have difficulty differentiating between true pathogens and colonizing organisms. BAL with quantitative culture was utilized in only 21 % of patients, but was not different between groups. A meta-analysis of four randomized controlled trials assessing invasive approaches to the diagnosis of VAP (confirmed bronchoscopically in 44–69 %) found that invasive sampling did not alter mortality, but did lead to modifications in the antibiotic regimen (nearly three times more likely in patients with invasive cultures) [26].
We attempted to determine factors which differentiated patients in the ClinVAPonly group who had ≤8 versus >8 days of VAP directed antibiotics, but the only significant difference was older age in the group treated for longer duration. Nonsignificant, but potentially relevant differences between shorter and longer treatment in this group included lower admission GCS and higher rate of MDROs on sputum culture in those receiving >8 versus ≤8 days of treatment (median GCS 7 vs. 9 and 19.1 vs. 6.7 %, respectively). The CPIS at initial diagnosis did not appear to drive therapeutic decisions (median CPIS 3: >8 days treatment vs. 4: <8 days treatment). Since these patients did not meet CDC criteria for VAP, the possibility of colonization must be acknowledged.
Many of the above weaknesses in diagnosing VAP have been addressed by the new CDC definition for VAP which removed findings such as radiographic infiltrates, altered mental status, fever and leukocytosis/leukopenia in favor of clinical findings which must persist over time and an emphasis on quantitative sputum specimens, and positive cultures which exclude mixed respiratory or oral flora, Candida, coagulase-negative Staphylococcus and Enterococcus [27]. If we remove just the above organisms from our VAP-treated group, the number of positive cases would drop to 44/57 (77.2 %) in the ClinVAPonly group and to 21/26 (80.77 %) cases in the SurVAP group. Moreover, reassessment of clinical features over the first 72 h of treatment prior to assuming the diagnosis and discontinuation of antibiotics when clinical findings and laboratory data are not congruent is expected to further reduce variability between clinician and surveillance-directed diagnosis [28, 29].
The following limitations of this study were identified. A small number of patients with confirmed VAP were identified in this 18-month assessment. This may have led to type II errors. Generalizability of our results may be limited to neurological and neurosurgical patients with low level of consciousness at admission (median GCS was 8 and 9), a major risk factor for VAP. Although we prospectively identified and collected data as part of a surveillance program, we acknowledge that this method is not equivalent to the real-time decision making of the clinical team. We have previously noted that most sputum cultures in this NCCU were obtained from endotracheal aspirates and few samples were from the lower airway. Over-reliance on non-bronchoscopic sampling for bacterial culture may have influenced antibiotic prescribing and likely impacted VAP diagnosis in the ClinVAPonly group which had relatively low incidences of other criteria. Finally, administrative errors in counting antibiotic days by the treating team may have unintentionally increased treatment duration. We appealed to recommendations that standard duration of therapy for VAP is 7–8 days for most pathogens and usually 14 days for non-lactose fermenting gram-negative bacilli [8]. We accounted for the presence of other infections and MDROs. We acknowledge, however, that there exists uncertainty regarding the appropriate diagnostic approach, optimal duration of therapy and the role of combination therapy [30].
The inappropriate use of antibiotics is associated with higher cost of treatment, direct costs for daily use of the antibiotic and indirect costs, including laboratory costs to check serum levels (e.g., vancomycin) and costs associated with not infrequent systemic side effects. Current methods of avoiding inappropriate antibiotic use utilize antimicrobial stewardship programs to optimize antibiotic selection, dose and duration. Strategies such as preauthorization/formulary restriction and prospective audit with feedback are increasingly common [31]. De-escalation of empiric antimicrobial therapy on the basis of culture results and the clinical setting can increase efficacy in targeting causative pathogens [29]. To improve physician behavior and processes of care in our neuro-ICU, our hospital infectious disease service initiated a VAP order set for all ICUs which grants 3 days of empiric antibiotics for suspected VAP followed by mandatory reassessment upon which further antibiotics can only be continued with ID on-call team approval. Education and implementation of miniBAL procedures were initiated to improve the rate of obtaining lower lung samples for culture.
Conclusion
Despite the absence of a gold-standard VAP definition, stricter adherence to a combination of pulmonary features of VAP, namely change in sputum character, tachypnea, oxygen desaturation and persistent infiltrate on chest X-ray, along with more frequent use of BAL with quantitative culture, may raise the threshold for diagnosing VAP and prevent initiating prolonged courses of antibiotics with associated increased health costs, risk of adverse reactions and selection of drug-resistant organisms. Judicious use of antibiotics and appropriate duration of treatment of VAP can potentially lead to significant cost savings and appears to be justifiable given that prolonged antibiotic prescribing does not appear to be associated with identifiable clinical parameters.
References
Hunter JD. Ventilator associated pneumonia. BMJ. 2012;344:e3325.
Vincent J, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, et al. The prevalence of nosocomial infection in intensive care units in Europe in intensive care (EPIC) study. JAMA. 1995;274:639–44.
Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–21.
Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637–57.
Amin A. Clinical and economic consequences of ventilator-associated pneumonia. Clin Infect Dis. 2009;49:S36–43.
Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2012, device-associated module. Am J Infect Control. 2013;41:1148–66.
Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–93.
Kuusinen P, Ala-Kokko T, Jartti A, Ahvenjarvi L, Saynajakangas P, Ohtonen P, et al. The occurrence of pneumonia diagnosis among neurosurgical patients: the definition matters. Neurocrit Care. 2012;16:123–9.
Swoboda SM, Dixon T, Lipsett PA. Can the clinical pulmonary infection score impact ICU antibiotic days? Surg Infect (Larchmt). 2006;7(4):331–9.
Nussenblatt V, Avdic E, Berenholtz S, Daugherty E, Hadhazy E, Lipsett PA, et al. Ventilator-associated pneumonia: inappropriate diagnosis and treatment is common in medical and surgical intensive care units. Infect Control Hosp Epidemiol. 2014;35(3):278–84.
Horan TC, Andrus M, Dudeck M. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32.
http://www.cdc.gov/nhsn/pdfs/pscmanual/17pscnosinfdef_current.pdf.
Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, et al. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–98.
File TM. Recommendations for treatment of hospital-acquired and ventilator-associated pneumonia: review of recent international guidelines. Clin Infect Dis. 2010;51:S42–7.
Naidech AM, Liebling SM, Duran IM, Moore MJ, Wunderink RG, Zembower TR. Reliability of the validated clinical diagnosis of pneumonia on validated outcomes after intracranial hemorrhage. J Crit Care. 2012;27(527):e7–11.
Klompas M. Interobserver variability in ventilator-associated pneumonia surveillance. Am J Infect Control. 2010;38:237–9.
Petersen IS, Aru A, Skødt V, Behrendt N, Bols B, Kiss K, et al. Evaluation of pneumonia diagnosis in intensive care patients. Scand J Infect Dis. 1999;31:299–303.
Fagon JY, Chastre J, Hance AJ, Domart Y, Trouillet JL, Gibert C. Evaluation of clinical judgment in the identification and treatment of nosocomial pneumonia in ventilated patients. Chest. 1993;103:547–53.
Luna CM, Vujacich P, Niederman MS, Vay C, Gherardi C, Matera J, et al. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest. 1997;111:676–85.
Alvarez-Lerma F, ICU-Acquired Pneumonia Study Group. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensiv Care Med. 1996;22:387–94.
Trouillet JL, Chastre J, Vuagnat A, Joly-Guillou ML, Combaux D, Dombret MC, et al. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am J Respir Crit Care Med. 1998;157:531–9.
Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest. 1993;104:1230–5.
Wunderink G, Wokknberg S, Zeiss J, Day M, Lacher A. The radiologic diagnosis proven ventilator-associated of autopsy—pneumonia. Chest. 1992;101:458–63.
American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416.
Shorr AF, Sherner JH, Jackson WL, Kollef MH. Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit Care Med. 2005;33:46–53.
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–11.
Masterton RG. Antibiotic de-escalation. Crit Care Clin. 2011;27:149–62.
Park DR. Antimicrobial treatment of ventilator-associated pneumonia. Respir Care. 2005;50:932–52.
Griffith M, Postelnick M, Scheetz M. Antimicrobial stewardship programs: methods of operation and suggested outcomes. Expert Rev Anti Infect Ther. 2012;10:63–73.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalanuria, A.A., Fellerman, D., Nyquist, P. et al. Variability in Diagnosis and Treatment of Ventilator-Associated Pneumonia in Neurocritical Care Patients. Neurocrit Care 23, 44–53 (2015). https://doi.org/10.1007/s12028-015-0109-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0109-x