Abstract
Purpose
Treatment with dopamine agonists (DA) is highly effective in patients with prolactinomas. In selected patients, discontinuation of DA after several years of successful treatment is possible, however, hyperprolactinemia recurs in 60–80% of them. It is unclear what is the clinical significance of these recurrences and hence, whether or not reinitiation of therapy is necessary.
Objectives
To evaluate the recurrence rate in prolactinoma patients after DA withdrawal and the necessity to restart treatment.
Methods
Patients with >2 years of treatment with cabergoline (CBG) who achieved normoprolactinemia and a > 50% reduction in tumor size were included. DA dose was down titrated until withdrawal. Basal tumor size, as well as PRL and gonadal steroid levels were recorded at diagnosis, at withdrawal of DA and every 3–6 months for 1–3 years.
Results
Fifty patients were included (38 women, 34 macroprolactinomas). After withdrawal, 34 (68%) presented recurrence of hyperprolactinemia. PRL levels <5 ng/mL at the time of withdrawal predicted remission (sensitivity 76%, specificity of 63%). CBG was restarted in eight patients (23%) because of the presence of hypogonadism. CBG was withheld in the remaining 26, based on the following arguments: (1) premenopausal women without biochemical hypogonadism, (54%); (2) asymptomatic men under 65 without biochemical hypogonadism (19%); (3) asymptomatic postmenopausal women (19%); (4) asymptomatic men over 65 (8%). After a median follow-up of 30 months, no increase in PRL levels or tumor growth was documented.
Conclusions
Biochemical recurrence in prolactinomas is very frequent, however, in only a few of these patients reinitiation of DA is necessary.
Similar content being viewed by others
Introduction
Prolactinoma is the most common type of secretory pituitary adenoma [1] with an approximate prevalence of 500 cases per million inhabitants and an annual incidence of 27 cases per 100,000 inhabitants. They are classified according to their size as microprolactinomas (<10 mm) and macroprolactinomas (>10 mm) [2]. Microprolactinomas are more frequently diagnosed in women, while macroprolactinomas are more prevalent in men [1]. For the past 50 years, the primary treatment of choice in prolactinomas has been pharmacological with dopamine agonists (DA) [3, 4]. DA act through specific Gi-protein-coupled receptors which are abundantly expressed by the tumoral lactotroph, potently inhibiting prolactin (PRL) gene expression, reducing cellular proliferation, and inducing vacuolization and apoptosis [4,5,6,7]. Treatment with DA results in PRL normalization and a > 50% reduction in tumor size in over 90% of patients with microprolactinomas, 70–80% with macroprolactinomas < 40 mm and in 55% of patients with tumors > 40 mm [4, 8,9,10]. The major drawback of DA treatment of prolactinomas is the need for life-long therapy [3, 11].
Discontinuation of DA after several years of successful treatment has been attempted since the late 1980s with recurrence rates between 65 and 80% [11,12,13]. According to a recent meta-analysis, factors associated with a successful withdrawal of DA include a normal PRL concentration, the absence of tumor remnant on MRI and the requirement of a relatively low DA dose [10, 12, 14, 15]. In most cases, recurrences after DA discontinuation are biochemical rather than clinical, and information regarding the long-term outcome of these patients is scarce [5, 11, 13, 16,17,18,19,20,21,22,23]. This study was designed to evaluate the long-term outcome of patients with prolactinomas in whom recurrence of hyperprolactinemia occurred after DA discontinuation, trying to identify factors associated with clinically significant relapses, and thus establishing real-life criteria that would justify the reinitiation of pharmacological treatment.
Patients and methods
Patient population
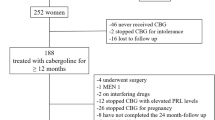
The study took place at the Prolactinoma Clinic of Hospital de Especialidades, Centro Médico Nacional Siglo XXI, whereby over 400 patients are treated and followed. Fifty consecutive patients identified between June 2015 and July 2019 were included according to the following criteria: (2) continuous treatment with cabergoline (CBG) for at least 2 years, (2) normalization of PRL concentrations, and (3) significant reduction in tumor size (≥50%) [3]. The study protocol consisted of a gradual downtitration of the CBG dose over 3–6 months until discontinuation. Once CBG was discontinued, patients were followed with periodic PRL determinations (every 3–6 months) and MRI (every 6 months during the first year and every 12 months later on). The decision to reinitiate CBG therapy was individually made taking into consideration the recurrence of hyperprolactinemia and re-growth of the adenoma, as well as the reappearance of galactorrhea and menstrual abnormalities in women and sexual dysfunction in males. The study was approved by our local ethics and scientific committees and each patient signed an informed consent upon enrollment.
Hormonal assays
PRL levels were measured by an electrochemiluminescence immunoassay “ECLIA” (ROCHE Diagnostics, IN, USA), calibrated with the 3rd IRP WHO Reference Standard 84/500, with a detection limit of 0.047 ng/mL, and intra- and inter-assay coefficients of variation of 4 and 5%, respectively. Macroprolactinemia was investigated in selected cases by means of polyethylene-glycol precipitation, as previously described [24]. When suspected, the hook effect was ruled out by performing the assay in 1:100 diluted serum samples. Hormones were determined by the next commercially available immunoassays: total testosterone (ECLIA, Elecsys, Roche Diagnostics, Switzerland, REF 122,11776061), TSH (Atellica TSH3-UL, Siemens, Germany), Free T4 (FT4) (Atellica FT4, Siemens, Germany) cortisol (Liaison assay, REF 313261, DiaSorin, Italy), GH (DiaSorin-Liaison assay, Italy, IRP WHO second 95/574), and IGF-1 (DiaSorin-Liaison chemiluminescent assay, Italy, IRP WHO second 02/254).
Magnetic resonance imaging
All patients had a gadolinium-enhanced pituitary MRI at diagnosis, at the withdrawal of CBG and several times upon follow-up. Tumor volume was calculated using the OsiriX (lite) DICOM viewer software (Geneva University Hospital).
Statistical methods
Quantitative variables are expressed as medians along with interquartile ranges (IQR), depending on their distribution. Proportions and frequencies were used for categorical variables. Differences in categorical variables between those who presented recurrence and those who did not were analyzed by the χ2 test or Fisher’s Exact test. Mann–Whitney U test was used for quantitative variables. We performed a multivariate analysis to find out factors associated with recurrence and ROC curves to identify the PRL level that predicts the persistence of remission. A p value of <0.05 was considered statistically significant. Data were analyzed using SPSS version 22 (SPSS Chicago, IL, USA).
Results
Fifty patients (38 women, 12 men) with a mean age of 43 ± 12 years were included in the study. Baseline characteristics at diagnosis are shown in Table 1. Sixteen patients (32%) harbored microadenomas (median largest diameter 8 mm [IQR 6–9]) and 34 harbored macroprolactinomas (median maximum largest diameter 22 mm [IQR 15–31]). The median PRL concentration at diagnosis was 200 ng/mL (IQR 149–230) and 644 ng/mL (IQR 367–1625), for patients with micro- and macroprolactinomas, respectively. All patients had an indication to start pharmacological treatment at diagnosis. All the patients with microprolactinoma were women and presented with menstrual disorders (100%), galactorrhea (87%), and infertility (25%). And in the patients with macroprolactinoma, compressive symptoms such as headache (75%) and visual field defects (44%) as well as central hypogonadism in women (100%) and men (88%) were present at diagnosis. All patients had been treated pharmacologically with CBG, at a median dose of 0.5 mg/week (IQR 0.25–0.75) immediately prior to discontinuation.
In 34 of the patients (68%) hyperprolactinemia recurred at a median of 3 months (IQR 2–6) after CBG discontinuation. Hyperprolactinemia recurrence occurred in 81% and 62% of the patients with microadenomas and macroadenomas, respectively. Recurring patients had significantly higher nadir PRL levels upon discontinuation (13.6 ng/mL vs. 4.3 ng/mL, p = 0.008) and a lower percent PRL reduction when on CBG (97% vs. 99.6%, p = 0.006). Recurring and nonrecurring patients did not differ regarding tumor size at diagnosis, percent tumor reduction, proportion with a visible tumor remnant, and CBG dose upon discontinuation (Table 2). When analyzing patients with micro- and macroprolactinomas separately, the difference between recurring and nonrecurring patients regarding nadir PRL levels upon discontinuation and the percent PRL reduction when on DA therapy persisted only in those with microprolactinomas (Table 3). Fifteen of the 34 patients with macroprolactinoma presented with a visual field defect due to chiasm compression; in 12, the visual defect resolved with DA treatment, whereas in the remaining 3 patients the visual field defect improved substantially (although a partial defect persisted), along with an excellent hormonal response and tumoral stability on imaging studies.
Multivariate analysis performed in the total group revealed that only the nadir PRL levels upon discontinuation were marginally associated with a higher hyperprolactinemia recurrence rate (OR 1.12, 95% CI 1.007–1.22). In the same multivariate analysis, adjusting for age, gender, and tumor size, the percent PRL reduction while on CBG therapy was associated with a lower risk of recurrence (OR 0.75, 95% CI 0.57–0.99). Receiver operating curves (ROC) of nadir PRL levels upon discontinuation showed that a concentration < 5 ng/mL was predictive of continuing remission with a sensitivity and specificity of 76% and 63%, respectively.
Of the 34 patients with hyperprolactinemia recurrence, CBG was resumed in only 8 (23%) because of the presence of clinical and biochemical evidence of hypogonadism. The decision not to restart CBG therapy in the remaining 26 patients was based on the following clinical judgment considerations (Table 4): (1) asymptomatic premenopausal women without clear-cut biochemical evidence of hypogonadism and not desiring pregnancy (n = 14); (2) asymptomatic men under age 65 without clear-cut biochemical evidence of hypogonadism (n = 5); (3) postmenopausal women without mass effect (n = 5); and (4) men over age 65 without mass effect (n = 2). Women included in category “a,” kept regular menstrual cycles, without galactorrhea despite a median PRL level upon recurrence of 68 ng/mL (IQR 54–124). In 8 of these 14 patients the presence of macroprolactinemia was documented at the time of recurrence by PEG precipitation (monomeric PRL recovery < 40%), whereas in two the recovery was 40–60%, indicating the coexistence of macroprolactinemia and monomeric hyperprolactinemia. It is highly relevant to note that after a median follow-up of 30 months (IQR 12–40) in the 26 untreated patients with recurrence of hyperprolactinemia, the PRL levels did not increase further and in no case was there any increase in the size of the tumor remnant.
Discussion
The major drawback of the pharmacological treatment of prolactinomas with DA is the need to continue it for life in the majority of patients [9, 16]. Most of the biological actions of DA on the tumoral lactotroph are cytostatic, although there is some experimental evidence supporting the existence of a cytocidal effect [4, 7]. Several published studies have addressed the issue of DA discontinuation in prolactinoma patients who have achieved normoprolactinemia and whose tumors have decreased considerably in size [8, 10, 12, 14, 16, 20, 21]. The recurrence rate of hyperprolactinemia ranges from 30 to 60% in microprolactinomas and from 50 to 75% in macroprolactinomas, and the best predictors of recurrence are an elevated nadir PRL level and the presence of a tumor remnant at withdrawal [11, 16, 19,20,21,22]. Although the recurrence rate found in our macroprolactinoma patients is similar to that reported by other authors, our patients with microprolactinomas had an unusually high rate of hyperprolactinemia recurrence. It must be pointed out, however, that the few microprolactinoma patients that are followed at our clinic are not of the common type as they usually require relatively higher CBG doses (>2 mg per week) in order to achieve a normal PRL level and a significant reduction in tumor size. Like in other series, a higher nadir PRL level and a lower percent PRL reduction at the time of CBG discontinuation were both associated with recurrence. However, when we analyzed micro- and macroprolactinoma patients separately these associations remained significant only in those with microprolactinomas. Hyperprolactinemia recurrence occurred within one year of DA withdrawal, which is in agreement with previously published series [8]. In a meta-analysis including 19 studies and encompassing over 740 patients in whom DA had been discontinued after the achievement of a normal PRL level, Dekkers et al., reported that the pooled proportion of subjects who remained normoprolactinemic was only 21% (95% CI 14–30%, I2 81%) [14]. In a more recent systematic review that analyzed 24 studies encompassing over 1100 patients, the proportion with persistent normoprolactinemia was 36.6% in a random effects model (95% CI 29.4–44.2%, I2 82.5%). The remission rate in this meta-analysis ranged from 15.1% in macroprolactinoma patients treated with bromocriptine to 40.8% in microprolactinoma patients treated with CBG [15]. The more frequent use of CBG rather than bromocriptine, a longer treatment period, the achievement of a PRL level below 5 ng/mL, the absence of a tumor remnant, and a better selection of patients are all associated with a better chance of a successful discontinuation [10].
In the majority of cases, hyperprolactinemia recurrence after DA discontinuation is of questionable clinical significance and the advantages of re-starting DA therapy have not been formally evaluated. In clinical practice the decision to reinstitute treatment with DA is based on clinical judgment. Issues that need to be taken into consideration include the risk of tumor re-growth with the potential of optic chiasm compression; the desire to become pregnant; the presence of symptoms and signs of hypogonadism; and the presence of macroprolactinemia. Hyperprolactinemia recurrence after DA discontinuation is rarely, if ever, accompanied by adenoma re-growth [6, 9, 17]. In fact, routine MRI follow-up after DA discontinuation is recommended only in patients with a rising PRL level Reinitiation of DA therapy is recommended in relapsing premenopausal, otherwise asymptomatic women who wish to become pregnant, because moderately elevated PRL levels may impair fertility in the absence of other biochemical or clinical evidence of hypogonadism [25]. Clinical and biochemical evidence of hypogonadism is undoubtedly an indication to resume DA treatment in premenopausal women, but it serves no purpose in postmenopausal women [26]. It is worth mentioning, however, that estrogen replacement therapy in such postmenopausal women could result in further increments in PRL levels. The coexistence of macroprolactinemia and real monomeric hyperprolactinemia deserves special consideration. Although macroprolactin is for the most part biologically inactive and the majority of patients with pure macroprolactinemia are eugonadal and should not have any fertility problems, in a small proportion of these subjects, true monomeric hyperprolactinemia coexists. In such a scenario the reinitiation of DA therapy seems reasonable, particularly in women who wish to become pregnant [26, 27]. The decision to restart DA therapy in relapsing men with evidence of hypogonadism is somewhat more difficult and has to be individualized taking into account not only the age of the patient but also issues such as sexual dysfunction as well as physical and psychological well-being.
Based on these considerations we decided not to restart CBG treatment in 14 asymptomatic premenopausal women without biochemical evidence of hypogonadism, who kept regular menstrual cycles and did not wish to become pregnant; in 5 asymptomatic postmenopausal women who were already hypogonadal; 5 asymptomatic men under the age of 65; and in 2 asymptomatic men over the age of 65. Interestingly, in 10 of the 14 premenopausal women the presence of macroprolactinemia was demonstrated, which by itself explained why they were not clinically or biochemically hypogonadal despite an elevated PRL level. At this point in the evolution of the patients, the indication for treatment was reevaluated, which allowed us to identify patients who could remain without treatment [3]. None of our 26 recurrence patients who did not resume CBG treatment showed tumor growth or further rise in PRL levels at follow-up.
In conclusion, we have confirmed that hyperprolactinemia recurrence after withdrawal of DA is very frequent yet, the reinitiation of treatment is necessary in only a minority of relatively young patients with clinical and biochemical evidence of hypogonadism, particularly women who desire to become pregnant. The risk of re-growth of tumor remnant does not seem to be an issue of concern. Long-term follow-up of these patients is required with periodical assessment of the gonadotropic axis, and in cases of a rising PRL level, MRI.
Data availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
A. Wong, J.A. Eloy, W.T. Couldwell, J.K. Liu, Update on prolactinomas. Part 1: clinical manifestations and diagnostic challenges. J. Clin. Neurosci. 22(10), 1562–1567 (2015)
V. Melgar, E. Espinosa, E. Sosa, M.J. Rangel, D. Cuenca, C. Ramirez et al. Current diagnosis and treatment of hyperprolactinemia. Rev. Med. Inst. Mex. Seguro Soc. 54(1), 111–121 (2016)
S. Melmed, F.F. Casanueva, A.R. Hoffman, D.L. Kleinberg, V.M. Montori, J.A. Schlechte et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96(2), 273–288 (2011)
A. Wong, J.A. Eloy, W.T. Couldwell, J.K. Liu, Update on prolactinomas. Part 2: treatment and management strategies. J. Clin. Neurosci. 22(10), 1568–1574 (2015)
M.D. Bronstein, Potential for long-term remission of microprolactinoma after withdrawal of dopamine-agonist therapy. Nat. Clin. Pract. Endocrinol. Metab. 2(3), 130–131 (2006)
M. Biswas, J. Smith, D. Jadon, P. McEwan, D.A. Rees, L.M. Evans et al. Long-term remission following withdrawal of dopamine agonist therapy in subjects with microprolactinomas. Clin. Endocrinol. 63(1), 26–31 (2005)
G. Kontogeorgos, E. Horvath, K. Kovacs, C. Coire, R.V. Lloyd, B.W. Scheithauer et al. Morphologic changes of prolactin-producing pituitary adenomas after short treatment with dopamine agonists. Acta Neuropathol. 111(1), 46–52 (2006)
P. Anagnostis, F. Adamidou, S.A. Polyzos, Z. Efstathiadou, E. Karathanassi, M. Kita, Long term follow-up of patients with prolactinomas and outcome of dopamine agonist withdrawal: a single center experience. Pituitary 15(1), 25–29 (2012)
S.C. Dogansen, O.S. Selcukbiricik, S. Tanrikulu, S. Yarman, Withdrawal of dopamine agonist therapy in prolactinomas: in which patients and when? Pituitary 19(3), 303–310 (2016)
P. Souteiro, S. Belo, D. Carvalho, Dopamine agonists in prolactinomas: when to withdraw? Pituitary 23(1), 38–44 (2020)
M. Teixeira, P. Souteiro, D. Carvalho, Prolactinoma management: predictors of remission and recurrence after dopamine agonists withdrawal. Pituitary 20(4), 464–70 (2017)
J. Hu, X. Zheng, W. Zhang, H. Yang, Current drug withdrawal strategy in prolactinoma patients treated with cabergoline: a systematic review and meta-analysis. Pituitary 18(5), 745–751 (2015)
R. Kwancharoen, R.S. Auriemma, G. Yenokyan, G.S. Wand, A. Colao, R. Salvatori, Second attempt to withdraw cabergoline in prolactinomas: a pilot study. Pituitary 17(5), 451–456 (2014)
O.M. Dekkers, J. Lagro, P. Burman, J.O. Jorgensen, J.A. Romijn, A.M. Pereira, Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 95(1), 43–51 (2010)
M.Y. Xia, X.H. Lou, S.J. Lin, Z.B. Wu, Optimal timing of dopamine agonist withdrawal in patients with hyperprolactinemia: a systematic review and meta-analysis. Endocrine 59(1), 50–61 (2018)
M.S. Huda, N.B. Athauda, M.M. The, P.V. Carroll, J.K. Powrie, Factors determining the remission of microprolactinomas after dopamine agonist withdrawal. Clin. Endocrinol. 72(4), 507–511 (2010)
J. Kharlip, R. Salvatori, G. Yenokyan, G.S. Wand, Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J. Clin. Endocrinol. Metabol. 94(7), 2428–2436 (2009)
L. Vilar, J.L. Albuquerque, P.S. Gadelha, F. Rangel Filho, A.M. Siqueira, M.M. da Fonseca et al. Second attempt of cabergoline withdrawal in patients with prolactinomas after a failed first attempt: is it worthwhile? Front. Endocrinol. 6, 11 (2015)
T.M. Barber, J. Kenkre, C. Garnett, R.V. Scott, J.V. Byrne, J.A. Wass, Recurrence of hyperprolactinaemia following discontinuation of dopamine agonist therapy in patients with prolactinoma occurs commonly especially in macroprolactinoma. Clin. Endocrinol. 75(6), 819–824 (2011)
A. Colao, A. Di Sarno, E. Guerra, R. Pivonello, P. Cappabianca, F. Caranci et al. Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin. Endocrinol. 67(3), 426–433 (2007)
M.J. Ji, J.H. Kim, J.H. Lee, J.H. Lee, Y.H. Kim, S.H. Paek et al. Best candidates for dopamine agonist withdrawal in patients with prolactinomas. Pituitary 20(5), 578–84. (2017)
E. Sala, P. Bellaviti Buttoni, E. Malchiodi, E. Verrua, G. Carosi, E. Profka et al. Recurrence of hyperprolactinemia following dopamine agonist withdrawal and possible predictive factors of recurrence in prolactinomas. J. Endocrinolog. Investig. 39(12), 1377–82. (2016)
S. Watanabe, H. Akutsu, S. Takano, T. Yamamoto, E. Ishikawa, H. Suzuki et al. Long-term results of cabergoline therapy for macroprolactinomas and analyses of factors associated with remission after withdrawal. Clin. Endocrinol. 86(2), 207–13. (2017)
A.O. Olukoga, J.W. Kane, Macroprolactinaemia: validation and application of the polyethylene glycol precipitation test and clinical characterization of the condition. Clin. Endocrinol. 51(1), 119–126 (1999)
K. Thirunavakkarasu, P. Dutta, S. Sridhar, L. Dhaliwal, G.R. Prashad, S. Gainder et al. Macroprolactinemia in hyperprolactinemic infertile women. Endocrine 44(3), 750–755 (2013)
S. Pekic, M. Medic Stojanoska, V. Popovic, Hyperprolactinemia/prolactinomas in the postmenopausal period: challenges in diagnosis and management. Neuroendocrinology 109(1), 28–33 (2019)
N. Hattori, T. Adachi, T. Ishihara, A. Shimatsu, The natural history of macroprolactinaemia. Eur. J. Endocrinol. 166(4), 625–629 (2012)
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S.-G.M., E.-C.E., R.-R.C., and M.-Z.V. The first draft of the paper was written by S.-E.E. and M.M. and all authors commented on previous versions of the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee from Hospital de Especialidades, UMAE Centro Médico Nacional Siglo XXI, IMSS, with registration number R-2018-3601-015.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Espinosa-Cárdenas, E., Sánchez-García, M., Ramírez-Rentería, C. et al. High biochemical recurrence rate after withdrawal of cabergoline in prolactinomas: is it necessary to restart treatment?. Endocrine 70, 143–149 (2020). https://doi.org/10.1007/s12020-020-02388-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02388-0