Abstract
Purpose
This study aimed to compare the nigella sativa vs. placebo effect on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome.
Methods
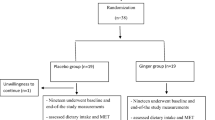
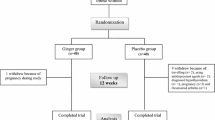
This randomized, double-blinded, placebo-controlled trial was conducted as a third-phase trial among 140 menopausal women within the age of 45–60 years old, who were suffering from metabolic syndrome and were assigned to receive 500 mg nigella sativa or placebo pill once daily. Anthropometric and biochemical parameters including body weight, waist circumference, serum lipid profile, fasting blood sugar, and HbA1C were measured at baseline and 8 weeks after administration the ingredient or placebo.
Results
In nigella sativa group, the serum markers such as low-density lipoprotein (115.1 ± 17.6 vs. 127.7 ± 12.6), triglyceride (158.3 ± 14.0 vs. 166.7 ± 16.0), total cholesterol (115.1 ± 17.6 vs. 127.7 ± 12.6), and fasting blood sugar (90.8 ± 16.9 vs. 113.7 ± 12.1) decreased significantly compared with the placebo (p < 0.001).
Conclusion
Administration of nigella sativa might be recommended for improving lipid profile and blood sugar in postmenopausal women with the metabolic syndrome.
Similar content being viewed by others
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
T.A. Takahashi, K.M. Johnson, Menopause. Med. Clin. N. Am. 99(3), 521–534 (2015). https://doi.org/10.1016/j.mcna.2015.01.006
M. Shirazi, N. Saedi, M. Shariat, F. Azadi, F.D. Tanha, Comparison of melissa with citalopram and placebo in treatment of sleep disorders in menopausal women: clinical trial. Tehran Univ. Med. J. 74(8), 562–568 (2016)
F. Davari-Tanha, M. Soleymani-Farsani, M. Asadi, M. Shariat, M. Shirazi, H. Hadizadeh, Comparison of citalopram and venlafaxine’s role in treating sleep disturbances in menopausal women, a randomized, double-blind, placebo-controlled trial. Arch. Gynecol. Obstet. 293(5), 1007–1013 (2016). https://doi.org/10.1007/s00404-015-3900-1
M. Ahuja, Age of menopause and determinants of menopause age: a PAN India survey by IMS. J. Midlife Health 7(3), 126–131 (2016). https://doi.org/10.4103/0976-7800.191012
J.C. Lovejoy, The menopause and obesity. Prim. Care 30(2), 317–325 (2003). https://doi.org/10.1016/s0095-4543(03)00012-5
A. Milewicz, Menopausal obesity and metabolic syndrome - PolSenior study. Minerva Endocrinol. 37(1), 93–101 (2012)
P.J. Miranda, R.A. DeFronzo, R.M. Califf, J.R. Guyton, Metabolic syndrome: definition, pathophysiology, and mechanisms. Am. Heart J. 149(1), 33–45 (2005). https://doi.org/10.1016/j.ahj.2004.07.013
R. Eshtiaghi, A. Esteghamati, M. Nakhjavani, Menopause is an independent predictor of metabolic syndrome in Iranian women. Maturitas 65(3), 262–266 (2010). https://doi.org/10.1016/j.maturitas.2009.11.004
A. Valdes, T. Bajaj, Estrogen therapy (StatPearls, Treasure Island, FL, 2020)
K. Magraith, B. Stuckey, An update on hormone therapy for menopause. Medicine Today 19(1), 41–44 (2018).
J. Woyka, Consensus statement for non-hormonal-based treatments for menopausal symptoms. Post Reprod. Health 23(2), 71–75 (2017). https://doi.org/10.1177/2053369117711646
D. Tonob, M.K. Melby, Broadening our perspectives on complementary and alternative medicine for menopause: a narrative review. Maturitas 99, 79–85 (2017). https://doi.org/10.1016/j.maturitas.2017.01.013
A.O. Bamosa, H. Kaatabi, F.M. Lebdaa, A.M. Elq, A. Al-Sultanb, Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharm. 54(4), 344–354 (2010)
A.M. Sabzghabaee, M. Dianatkhah, N. Sarrafzadegan, S. Asgary, A. Ghannadi, Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: a randomized, placebo controlled clinical trial. Med. Arch. 66(3), 198–200 (2012). https://doi.org/10.5455/medarh.2012.66.198-200
S. Rooney, M.F. Ryan, Effects of alpha-hederin and thymoquinone, constituents of Nigella sativa, on human cancer cell lines. Anticancer Res. 25(3B), 2199–2204 (2005)
A.N. Shuid, N. Mohamed, I.N. Mohamed, F. Othman, F. Suhaimi, E.S. Mohd Ramli, N. Muhammad, I.N. Soelaiman, Nigella sativa: a potential antiosteoporotic agent. Evid. Complement Altern. Med. 2012, 696230 (2012). https://doi.org/10.1155/2012/696230
P.L. Huang, A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2(5–6), 231–237 (2009). https://doi.org/10.1242/dmm.001180
A. Ahmad, A. Husain, M. Mujeeb, S.A. Khan, A.K. Najmi, N.A. Siddique, Z.A. Damanhouri, F. Anwar, A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac. J. Trop. Biomed. 3(5), 337–352 (2013). https://doi.org/10.1016/S2221-1691(13)60075-1
I. Meral, Z. Yener, T. Kahraman, N. Mert, Effect of Nigella sativa on glucose concentration, lipid peroxidation, anti-oxidant defence system and liver damage in experimentally-induced diabetic rabbits. J. Vet. Med. A Physiol. Pathol. Clin. Med. 48(10), 593–599 (2001). https://doi.org/10.1046/j.1439-0442.2001.00393.x
S. Parhizkar, L.A. Latiff, A. Parsa, Effect of Nigella sativa on reproductive system in experimental menopause rat model. Avicenna J. Phytomed. 6(1), 95–103 (2016)
M. Molaie, B. Darvishi, Z. Jafari Azar, M. Shirazi, G. Amin, S. Afshar, Effects of a combination of Nigella sativa and Vitex agnus-castus with citalopram on healthy menopausal women with hot flashes: results from a subpopulation analysis. Gynecol. Endocrinol. 35(1), 58–61 (2019). https://doi.org/10.1080/09513590.2018.1499086
A. Shekhi-Karzaki, Pharmacognosy of Nigella sativa and components of essence by GC-MS method. Dissertation for doctorate. Tehran Univ. Med. Sec. (2000)
J.M. Jellin, P. Gregory, F. Batz, K. Hitchens, Natural Medicines Comprehensive Database, 3d ed. Compiled and edited by J.M. Jellin, P. Gregory, F. Batz, K. Hitchens, S. Burson, K. Shaver, K. Palacioz. 1,530 p (Therapeutic Research, Stockton, CA, 2000)
A. Zaoui, Y. Cherrah, N. Mahassini, K. Alaoui, H. Amarouch, M. Hassar, Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 9(1), 69–74 (2002). https://doi.org/10.1078/0944-7113-00084
B.H. Ali, G. Blunden, Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 17(4), 299–305 (2003). https://doi.org/10.1002/ptr.1309
Author information
Authors and Affiliations
Contributions
M.S.: project development and manuscript writing. M.G.: data collection and management and manuscript editing. E.F.: manuscript editing and data management. F.K.: data management. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
The protocol of the study was approved by the ethical committee of Tehran University of Medical Sciences. The participants subsequently submitted a written consent form to participate in the trial. This trial was conducted according to the principles of the Helsinki Declaration.
Consent for publication
Written consent was signed upon admission by the patient included in this study to use their information in research studies and as available for review. No personal information had been published and the identity of the participants had not transpired.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shirazi, M., Khodakarami, F., Feizabad, E. et al. The effects of nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine 69, 49–52 (2020). https://doi.org/10.1007/s12020-020-02265-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02265-w