Abstract
Purpose
Current reference methods for measuring glucose effectiveness (GE) are the somatostatin pancreatic glucose clamp and minimal model analysis of frequently sampled intravenous glucose tolerance test (FSIVGTT), both of which are laborious and not feasible in large epidemiological studies. Consequently, surrogate indices derived from an oral glucose tolerance test (OGTT) to measure GE (oGE) have been proposed and used in many studies. However, the predictive accuracy of these surrogates has not been formally validated. In this study, we used a calibration model analysis to evaluate the accuracy of surrogate indices to predict GE from the reference FSIVGTT (SgMM).
Methods
Subjects (n = 123, mean age 48 ± 11 years; BMI 35.9 ± 7.3 kg/m2) with varying glucose tolerance (NGT, n = 37; IFG/IGT, n = 78; and T2DM, n = 8) underwent FSIVGTT and OGTT on two separate days. Predictive accuracy was assessed by both root mean squared error (RMSE) of prediction and leave-one-out cross-validation-type RMSE of prediction (CVPE).
Results
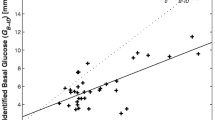
As expected, insulin sensitivity, SgMM, and oGE were reduced in subjects with T2DM and IFG/IGT when compared with NGT. Simple linear regression analyses revealed a modest but significant relationship between oGE and SgMM (r = 0.25, p < 0.001). However, using calibration model, measured SgMM and predicted SgMM derived from oGE were modestly correlated (r = 0.21, p < 0.05) with the best fit line suggesting poor predictive accuracy. There were no significant differences in CVPE and RMSE among the surrogates, suggesting similar predictive ability.
Conclusions
Although OGTT-derived surrogate indices of GE are convenient and feasible, they have limited ability to robustly predict GE.


Similar content being viewed by others
References
J. Tonelli, P. Kishore, D.E. Lee, M. Hawkins, The regulation of glucose effectiveness: how glucose modulates its own production. Curr. Opin. Clin. Nutr. Metab. Care. 8(4), 450–456 (2005)
J.D. Best, S.E. Kahn, M. Ader, R.M. Watanabe, T.C. Ni, R.N. Bergman, Role of glucose effectiveness in the determination of glucose tolerance. Diabetes Care 19(9), 1018–1030 (1996)
S.E. Kahn, R.L. Prigeon, D.K. McCulloch, E.J. Boyko, R.N. Bergman, M.W. Schwartz, J.L. Neifing, W.K. Ward, J.C. Beard, J.P. Palmer et al. The contribution of insulin-dependent and insulin-independent glucose uptake to intravenous glucose tolerance in healthy human subjects. Diabetes 43(4), 587–592 (1994)
C. Lorenzo, L.E. Wagenknecht, A.J. Karter, A.J. Hanley, M.J. Rewers, S.M. Haffner, Cross-sectional and longitudinal changes of glucose effectiveness in relation to glucose tolerance: the insulin resistance atherosclerosis study. Diabetes Care 34(9), 1959–1964 (2011)
B.C. Martin, J.H. Warram, A.S. Krolewski, R.N. Bergman, J.S. Soeldner, C.R. Kahn, Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340(8825), 925–929 (1992)
M.F. Nielsen, R. Basu, S. Wise, A. Caumo, C. Cobelli, R.A. Rizza, Normal glucose-induced suppression of glucose production but impaired stimulation of glucose disposal in type 2 diabetes: evidence for a concentration-dependent defect in uptake. Diabetes 47(11), 1735–1747 (1998)
A. Basu, C. Dalla Man, R. Basu, G. Toffolo, C. Cobelli, R.A. Rizza, Effects of type 2 diabetes on insulin secretion, insulin action, glucose effectiveness, and postprandial glucose metabolism. Diabetes Care 32(5), 866–872 (2009)
M.B. Egede, J.E. Henriksen, T.T. Durck, K. Levin, C. Rantzau, G. Ward, H. Beck-Nielsen, F.P. Alford, Glucose effectiveness in nondiabetic relatives: dysglycemia and beta-cell function at 10 years. J. Clin. Endocrinol. Metab. 99(4), 1420–1424 (2014). https://doi.org/10.1210/jc.2013-3273
K. Karstoft, M.A. Clark, I. Jakobsen, S.H. Knudsen, G. van Hall, B.K. Pedersen, T.P.J. Solomon, Glucose effectiveness, but not insulin sensitivity, is improved after short-term interval training in individuals with type 2 diabetes mellitus: a controlled, randomised, crossover trial. Diabetologia 60(12), 2432–2442 (2017). https://doi.org/10.1007/s00125-017-4406-0
J.E. Henriksen, F. Alford, G. Ward, P. Thye-Ronn, K. Levin, O. Hother-Nielsen, C. Rantzau, R. Boston, H. Beck-Nielsen, Glucose effectiveness and insulin sensitivity measurements derived from the non-insulin-assisted minimal model and the clamp techniques are concordant. Diabetes Metab. Res. Rev. 26(7), 569–578 (2010)
S. Nagasaka, I. Kusaka, K. Yamashita, Y. Funase, K. Yamauchi, M. Katakura, S. Ishibashi, T. Aizawa, Index of glucose effectiveness derived from oral glucose tolerance test. Acta Diabetol. 49(Suppl 1), S195–S204 (2012)
R. Weiss, S.N. Magge, N. Santoro, C. Giannini, R. Boston, T. Holder, M. Shaw, E. Duran, K.J. Hershkop, S. Caprio, Glucose effectiveness in obese children: relation to degree of obesity and dysglycemia. Diabetes Care 38(4), 689–695 (2015)
American Diabetes, A, Diagnosis and classification of diabetes mellitus. Diabetes Care 34(Suppl 1), S62–S69 (2011). https://doi.org/10.2337/dc11-S062
S.J. Healy, K. Osei, T. Gaillard, Comparative study of glucose homeostasis, lipids and lipoproteins, HDL functionality, and cardiometabolic parameters in modestly severely obese African Americans and White Americans with prediabetes: implications for the metabolic paradoxes. Diabetes Care 38(2), 228–235 (2015). https://doi.org/10.2337/dc14-1803
R. Muniyappa, V. Sachdev, S. Sidenko, M. Ricks, D.C. Castillo, A.B. Courville, A.E. Sumner, Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am. J. Physiol. Endocrinol. Metab. 302(2), E218–E225 (2012). https://doi.org/10.1152/ajpendo.00434.2011
S. Soonthornpun, W. Setasuban, A. Thamprasit, W. Chayanunnukul, C. Rattarasarn, A. Geater, Novel insulin sensitivity index derived from oral glucose tolerance test. J. Clin. Endocrinol. Metab. 88(3), 1019–1023 (2003). https://doi.org/10.1210/jc.2002-021127
C. Giannini, R. Weiss, A. Cali, R. Bonadonna, N. Santoro, B. Pierpont, M. Shaw, S. Caprio, Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 61(3), 606–614 (2012). https://doi.org/10.2337/db11-1111
I. Aloulou, J.F. Brun, J. Mercier, Evaluation of insulin sensitivity and glucose effectiveness during a standardized breakfast test: comparison with the minimal model analysis of an intravenous glucose tolerance test. Metabolism 55(5), 676–690 (2006). https://doi.org/10.1016/j.metabol.2006.01.002
J.F. Brun, E. Ghanassia, C. Fedou, S. Bordenave, E. Raynaud de Mauverger, J: Mercier, Assessment of insulin sensitivity (S I) and glucose effectiveness (S G) from a standardized hyperglucidic breakfast test in type 2 diabetics exhibiting various levels of insulin resistance. Acta Diabetol. 50(2), 143–153 (2013). https://doi.org/10.1007/s00592-010-0232-2
R. Muniyappa, B.A. Irving, U.S. Unni, W.M. Briggs, K.S. Nair, M.J. Quon, A.V. Kurpad, Limited predictive ability of surrogate indices of insulin sensitivity/resistance in Asian-Indian men. Am. J. Physiol. Endocrinol. Metab. 299(6), E1106–E1112 (2010). https://doi.org/10.1152/ajpendo.00454.2010
P. Singal, R. Muniyappa, R. Chisholm, G. Hall, H. Chen, M.J. Quon, K.J. Mather, Simple modeling allows prediction of steady-state glucose disposal rate from early data in hyperinsulinemic glucose clamps. Am. J. Physiol. Endocrinol. Metab. 298(2), E229–E236 (2010). https://doi.org/10.1152/ajpendo.00603.2009
M. Hawkins, I. Gabriely, R. Wozniak, K. Reddy, L. Rossetti, H. Shamoon, Glycemic control determines hepatic and peripheral glucose effectiveness in type 2 diabetic subjects. Diabetes 51(7), 2179–2189 (2002)
K. Osei, D.A. Cottrell, Minimal model analyses of insulin sensitivity and glucose-dependent glucose disposal in black and white Americans: a study of persons at risk for type 2 diabetes. Eur. J. Clin. Invest. 24(12), 843–850 (1994)
A.G. Amoah, S.K. Owusu, O.M. Ayittey, D.P. Schuster, K. Osei, Minimal model analyses of beta cell secretion, insulin sensitivity and glucose effectiveness in glucose tolerant, non-diabetic first-degree relatives of Ghanaian patients with type 2 diabetes and healthy control subjects. Ethn. Dis. 11(2), 201–210 (2001)
K. Osei, D.A. Cottrell, M.M. Orabella, Insulin sensitivity, glucose effectiveness, and body fat distribution pattern in nondiabetic offspring of patients with NIDDM. Diabetes Care 14(10), 890–896 (1991)
R.A. DeFronzo, Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37(6), 667–687 (1988)
C. Bogardus, S. Lillioja, B.V. Howard, G. Reaven, D. Mott, Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J. Clin. Invest. 74(4), 1238–1246 (1984). https://doi.org/10.1172/JCI111533
P. Vuguin, A.B. Sopher, H. Roumimper, V. Chin, M. Silfen, D.J. McMahon, I. Fennoy, S.E. Oberfield, Alterations in glucose effectiveness and insulin dynamics: polycystic ovary syndrome or body mass index. Horm. Res. Paediatr. 87(6), 359–367 (2017). https://doi.org/10.1159/000471804
Funding
This work was supported by the Intramural Research Programs of NIDDK (R.M.), American Diabetes Association Clinical and Translational Award Number: 0-11-CT-39, and Award Number UL1RR025755 from the National Center for Research Resources, funded by the Office of the Director, National Institutes of Health (NIH) and supported by the NIH Roadmap for Medical Research.
Author contributions
R.M. conceived and designed the study, acquired and analyzed data, drafted and reviewed the manuscript. M.G., S.S., S.G., and B.S.A. analyzed data, drafted, and reviewed the manuscript. R.M. and S.A. performed the statistical analyses and drafted the manuscript T.R.G. and K.O. designed the study, acquired data, and drafted and reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants in the study.
Rights and permissions
About this article
Cite this article
Glicksman, M., Grewal, S., Sortur, S. et al. Assessing the predictive accuracy of oral glucose effectiveness index using a calibration model. Endocrine 63, 391–397 (2019). https://doi.org/10.1007/s12020-018-1804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-018-1804-0