Abstract
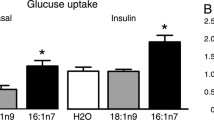
Decreases in serum testosterone concentrations in aging men are associated with metabolic disorders. Testosterone has been reported to increase GLUT4-dependent glucose uptake in skeletal muscle cells and cardiomyocytes. However, studies on glucose uptake occurring in response to testosterone stimulation in adipocytes are currently not available. This study was designed to determine the effects of testosterone on glucose uptake in adipocytes. Glucose uptake was assessed with 2-[3H] deoxyglucose in 3T3-L1 adipocytes. GLUT4 translocation was evaluated in plasma membrane (PM) sheets and PM fractions by immunofluorescence and immunoblotting, respectively. Activation of GLUT4 translocation-related protein kinases, including Akt, AMPK, LKB1, CaMKI, CaMKII, and Cbl was followed by immunoblotting. Expression levels of androgen receptor (AR) mRNA and AR translocation to the PM were assessed by real-time RT-PCR and immunoblotting, respectively. The results showed that both high-dose (100 nM) testosterone and testosterone-BSA increased glucose uptake and GLUT4 translocation to the PM, independently of the intracellular AR. Testosterone and testosterone-BSA stimulated the phosphorylation of AMPK, LKB1, and CaMKII. The knockdown of LKB1 by siRNA attenuated testosterone- and testosterone-BSA-stimulated AMPK phosphorylation and glucose uptake. These results indicate that high-dose testosterone and testosterone-BSA increase GLUT4-dependent glucose uptake in 3T3-L1 adipocytes by inducing the LKB1/AMPK signaling pathway.






Similar content being viewed by others
Abbreviations
- AMPK:
-
5′ adenosine monophosphate-activated protein kinase
- ANOVA:
-
One-way analysis of variance
- AR:
-
Androgen receptor
- BSA:
-
Bovine serum albumin
- CaMKI:
-
Ca2+/calmodulin-dependent protein kinase I
- CaMKII:
-
Ca2+/calmodulin-dependent protein kinase II
- CaMKKβ:
-
Ca2+/calmodulin-dependent protein kinase kinase β
- 2-DG:
-
2-deoxy-d-glucose
- FCS:
-
Fetal calf serum
- GLUT4:
-
Glucose transporter 4
- 3H-2DG:
-
2-[3H] deoxyglucose
- KPRH:
-
Krebs-Ringer-Phosphate-Hepes
- LKB1:
-
Liver kinase B1
- PI3K:
-
Phosphatidylinositol-3 kinase
- PM:
-
Plasma membrane
References
R.A. DeFronzo, Lilly Lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37, 667–687 (1988)
A.H. Khan, J.E. Pessin, Insulin regulation of glucose uptake: a complex interplay of intracellular signalling pathways. Diabetologia 45, 1475–1483 (2002)
S.H. Chiang, C.A. Baumann, M. Kanzaki, D.C. Thurmond, R.T. Watson, C.L. Neudauer, I.G. Macara, J.E. Pessin, A.R. Saltiel, Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948 (2001)
H.F. Kramer, C.A. Witczak, N. Fujii, N. Jessen, E.B. Taylor, D.E. Arnolds, K. Sakamoto, M.F. Hirshman, L.J. Goodyear, Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes 55, 2067–2076 (2006)
J.W. Ryder, A.V. Chibalin, J.R. Zierath, Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol. Scand. 171, 249–257 (2001)
T. Imamura, P. Vollenweider, K. Egawa, M. Clodi, K. Ishibashi, N. Nakashima, S. Ugi, J.W. Adams, J.H. Brown, J.M. Olefsky, G alpha-q/11 protein plays a key role in insulin-induced glucose transport in 3T3-L1 adipocytes. Mol. Cell. Biol. 19, 6765–6774 (1999)
R.R. Russell, R. Bergeron, G.I. Shulman, L.H. Young, Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am. J. Physiol. 277, H643–H649 (1999)
Y. Shen, N. Honma, K. Kobayashi, L.N. Jia, T. Hosono, K. Shindo, T. Ariga, T. Seki, Cinnamon extract enhances glucose uptake in 3T3-L1 adipocytes and C2C12 myocytes by inducing LKB1-AMP-activated protein kinase signaling. PLoS ONE 9, 1–9 (2014)
L. Wang, H. Hayashi, K. Kishi, L. Huang, A. Hagi, K. Tamaoka, P.T. Hawkins, Y. Ebina, Gi-mediated translocation of GLUT4 is independent of p85/p110α and p110γ phosphoinositide 3-kinases but might involve the activation of Akt kinase. Biochem. J. 345, 543–555 (2000)
J.R. Wu-Wong, C.E. Berg, J. Wang, W.J. Chiou, B. Fissel, Endothelin stimulates glucose uptake and GLUT4 translocation via activation of endothelin ET(A) receptor in 3T3-L1 adipocytes. J. Biol. Chem. 274, 8103–8110 (1999)
D.E. Laaksonen, L. Niskanen, K. Punnonen, K. Nyyssönen, T.P. Tuomainen, V.P. Valkonen, R. Salonen, J.T. Salonen, Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 27, 1036–1041 (2004)
M. Fukui, J. Soh, M. Tanaka, Y. Kitagawa, G. Hasegawa, T. Yoshikawa, T. Miki, N. Nakamura, Low serum testosterone concentration in middle-aged men with type 2 diabetes. Endocr. J. 54, 871–877 (2007)
A. Rodriguez, D.C. Muller, E.J. Metter, M. Maggio, S.M. Harman, M.R. Blackman, R. Andres, Aging, androgens, and the metabolic syndrome in a longitudinal study of aging. J. Clin. Endocrinol. Metab. 92, 3568–3572 (2007)
A. Holmäng, P. Björntorp, The effects of testosterone on insulin sensitivity in male rats. Acta Physiol. Scand. 146, 505–510 (1992)
T. Muthusamy, P. Murugesan, K. Balasubramanian, Sex steroids deficiency impairs glucose transporter 4 expression and its translocation through defective Akt phosphorylation in target tissues of adult male rat. Metabolism 58, 1581–1592 (2009)
E. Maneschi, A. Morelli, S. Filippi, I. Cellai, P. Comeglio, B. Mazzanti, T. Mello, A. Calcagno, E. Sarchielli, L. Vignozzi, F. Saad, R. Vettor, G.B. Vannelli, M. Maggi, Testosterone treatment improves metabolic syndrome-induced adipose tissue derangements. J. Endocrinol. 215, 347–362 (2012)
T. Senmaru, M. Fukui, H. Okada, Y. Mineoka, M. Yamazaki, M. Tsujikawa, G. Hasegawa, J. Kitawaki, H. Obayashi, N. Nakamura, Testosterone deficiency induces markedly decreased serum triglycerides, increased small dense LDL, and hepatic steatosis mediated by dysregulation of lipid assembly and secretion in mice fed a high-fat diet. Metabolism 62, 851–860 (2013)
K. Sato, M. Iemitsu, Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 294, 961–968 (2008)
C. Wilson, A. Contreras-Ferrat, N. Venegas, C. Osorio-Fuentealba, M. Pávez, K. Montoya, J. Durán, R. Maass, S. Lavandero, M. Estrada, Testosterone increases GLUT4-dependent glucose uptake in cardiomyocytes. J. Cell. Physiol. 228, 2399–2407 (2013)
L.J. Robinson, S. Pang, D.S. Harris, J. Heuser, D.E. James, Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP, insulin, and GTPγS and localization of GLUT4 to clathrin lattices. J. Cell Biol. 117, 1181–1196 (1992)
S. Nishiumi, H. Ashida, Rapid preparation of a plasma membrane fraction from adipocytes and muscle cells: application to detection of translocated glucose transporter 4 on the plasma membrane. Biosci. Biotechnol. Biochem. 71, 2343–2346 (2007)
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001)
C.A. Heinlein, C. Chang, The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 16, 2181–2187 (2002)
C.D. Foradori, M.J. Weiser, R.J. Handa, Non-genomic actions of androgens. Front. Neuroendocrinol. 29, 169–181 (2008)
Y.C. Chen, S.D. Lee, C.H. Kuo, L.T. Ho, The effects of altitude training on the AMPK-related glucose transport pathway in the red skeletal muscle both lean and obese Zuker rats. High Alt. Med. Biol. 12, 371–378 (2011)
M.J. Sanders, P.O. Grondin, B.D. Hegarty, M.A. Snowden, D. Carling, Investigating the mechanism for AMP activation of the AMP-activated protein kinase cascade. Biochem. J. 403, 139–148 (2007)
A. Gormand, E. Henriksson, K. Ström, T.E. Jensen, K. Sakamoto, O. Göransson, Regulation of AMP-activated protein kinase by LKB1 and CaMKK in adipocytes. J. Cell. Biochem. 112, 1364–1375 (2011)
S.S. Hook, A.R. Means, Ca(2+)/CaM-dependent kinases: from activation to function. Annu. Rev. Pharmacol. Toxicol. 41, 471–505 (2001)
A. Hudmon, H. Schulman, Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem. J. 364, 593–611 (2002)
K.J. McInnes, K.A. Brown, N.I. Hunger, E.R. Simpson, Regulation of LKB1 expression by sex hormones in adipocytes. Int. J. Obes (Lond) 36, 982–985 (2012)
K.J. McInnes, A. Corbould, E.R. Simpson, M.E. Jones, Regulation of adenosine 5′, monophosphate-activated protein kinase and lipogenesis by androgens contributes to visceral obesity in an estrogen-deficient state. Endocrinology 147, 5907–5913 (2006)
M. Fu, T. Sun, A.L. Bookout, M. Downes, R.T. Yu, R.M. Evans, D.J. Mangelsdorf, A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol. Endocrinol. 19, 2437–2450 (2005)
J.O. Lee, S.K. Lee, J.H. Kim, N. Kim, G.Y. You, J.W. Moon, S.J. Kim, S.H. Park, H.S. Kim, Metformin regulates glucose transporter 4 (GLUT4) translocation through AMP-activated protein kinase (AMPK)-mediated Cbl/CAP signaling in 3T3-L1 preadipocyte cells. J. Biol. Chem. 287, 44121–44129 (2012)
Acknowledgments
This study is supported by the Japan Society for the Promotion of Science KAKENHI (Grant Number 24591339 (M. Fukui)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Mitsuhashi, K., Senmaru, T., Fukuda, T. et al. Testosterone stimulates glucose uptake and GLUT4 translocation through LKB1/AMPK signaling in 3T3-L1 adipocytes. Endocrine 51, 174–184 (2016). https://doi.org/10.1007/s12020-015-0666-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0666-y