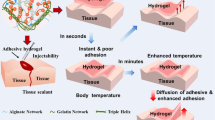
Abstract
Gelatin methacrylate (GelMA)-based hydrogels are gaining a great deal of attention as potentially implantable materials in tissue engineering applications because of their biofunctionality and mechanical tenability. Since different natural tissues respond differently to mechanical stresses, an ideal implanted material would closely match the mechanical properties of the target tissue. In this regard, applications employing GelMA hydrogels are currently limited by the low mechanical strength and biocompatibility of GelMA. Therefore, this review focuses on modifications made to GelMA hydrogels to make them more suitable for tissue engineering applications. A large number of reports detail rational synthetic processes for GelMA or describe the incorporation of various biomaterials into GelMA hydrogels to tune their various properties, e.g., physical strength, chemical properties, conductivity, and porosity, and to promote cell loading and accelerate tissue repair. A novel strategy for repairing tissue injuries, based on the transplantation of cell-loaded GelMA scaffolds, is examined and its advantages and challenges are summarized. GelMA-cell combinations play a critical and pioneering role in this process and could potentially accelerate the development of clinically relevant applications.




Similar content being viewed by others
References
Dey, J., Xu, H., Shen, J., Thevenot, P., Gondi, S., Nguyen, K., et al. (2008). Development of biodegradable crosslinked urethane-doped polyester elastomers. Biomaterials, 29, 4637–4649.
Guan, J., & Wagner, W. (2005). Synthesis, characterization and cytocompatibility of polyurethaneurea elastomers with designed elastase sensitivity. Biomacromolecules, 6, 2833–2842.
Bruggeman, J., de Bruin, B., Bettinger, C., & Langer, R. (2008). Biodegradable poly(polyol sebacate) polymers. Biomaterials, 29, 4726–4735.
Bettinger, C., Bruggeman, J., Borenstein, J., & Langer, R. (2008). Amino alcohol-based degradable poly(ester amide) elastomers. Biomaterials, 29, 2315–2325.
Van Vlierberghe, S., Dubruel, P., & Schacht, E. (2011). Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules, 12, 1387–1408.
Wichterle, O., & LÍM, D. (1960). Hydrophilic gels for biological use. Nature, 185, 117–118.
Serafim, A., Tucureanu, C., Petre, D.-G., Dragusin, D.-M., Salageanu, A., Van Vlierberghe, S., et al. (2014). One-pot synthesis of superabsorbent hybrid hydrogels based on methacrylamide gelatin and polyacrylamide. Effortless control of hydrogel properties through composition design. New Journal of Chemistry, 38, 3112–3126.
Lee, K. Y., & Mooney, D. J. (2001). Hydrogels for Tissue Engineering. Chemical Reviews, 101, 1869–1880.
Khademhosseini, A., Vacanti, J. P., & Langer, R. (2009). Progress in tissue engineering. Scientific American, 300, 64–71.
Annabi, N., Nichol, J., Zhong, X., Ji, C., Koshy, S., Khademhosseini, A., et al. (2010). Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Engineering. Part B, Reviews, 16, 371–383.
Li, Y., Rodrigues, J., & Tomás, H. (2012). Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chemical Society Reviews, 41, 2193–2221.
Pérez, R., Won, J., Knowles, J., & Kim, H. (2013). Naturally and synthetic smart composite biomaterials for tissue regeneration. Advanced Drug Delivery Reviews, 65, 471–496.
Van Den Bulcke, A., Bogdanov, B., De Rooze, N., Schacht, E., Cornelissen, M., & Berghmans, H. (2000). Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules, 1, 31–38.
Di Lullo, G. A., Sweeney, S. M., Korkko, J., Ala-Kokko, L., & San Antonio, J. D. (2002). Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. The Journal of Biological Chemistry, 277, 4223–4231.
Rahali, K., Ben Messaoud, G., Kahn, C., Sanchez-Gonzalez, L., Kaci, M., Cleymand, F., Fleutot, S., Linder, M., Desobry, S., & Arab-Tehrany, E. (2017). Synthesis and characterization of Nanofunctionalized gelatin methacrylate hydrogels. International Journal of Molecular Sciences, 18.
Lynn, A. K., Yannas, I. V., & Bonfield, W. (2004). Antigenicity and immunogenicity of collagen. Journal of biomedical materials research Part B, Applied biomaterials, 71, 343–354.
Shirahama, H., Lee, B., Tan, L., & Cho, N. (2016). Precise tuning of facile one-Pot gelatin Methacryloyl (GelMA) synthesis. Scientific Reports, 6, 31036.
Galis, Z., & Khatri, J. (2002). Matrix metalloproteinases in vascular remodeling and atherogenesis: The good, the bad, and the ugly. Circulation Research, 90, 251–262.
Nichol, J., Koshy, S., Bae, H., Hwang, C., Yamanlar, S., & Khademhosseini, A. (2010). Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials, 31, 5536–5544.
Van den Steen, P., Dubois, B., Nelissen, I., Rudd, P., Dwek, R., & Opdenakker, G. (2002). Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Critical Reviews in Biochemistry and Molecular Biology, 37, 375–536.
Liu, Y., & Chan-Park, M. (2010). A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture. Biomaterials, 31, 1158–1170.
Yue, K., Trujillo-de Santiago, G., Alvarez, M., Tamayol, A., Annabi, N., & Khademhosseini, A. (2015). Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials, 73, 254–271.
Powell, M. (1987). Stability of lidocaine in aqueous solution: Effect of temperature, pH, buffer, and metal ions on amide hydrolysis. Pharmaceutical Research, 4, 42–45.
Ratcliffe, J., Hunneyball, I., Smith, A., Wilson, C., & Davis, S. (1984). Preparation and evaluation of biodegradable polymeric systems for the intra-articular delivery of drugs. The Journal of Pharmacy and Pharmacology, 36, 431–436.
Knopf-Marques, H., Barthes, J., Wolfova, L., Vidal, B., Koenig, G., Bacharouche, J., Francius, G., Sadam, H., Liivas, U., Lavalle, P., & Vrana, N. E. (2017). Auxiliary biomembranes as a directional delivery system to control biological events in cell-laden tissue-engineering scaffolds. ACS Omega, 2, 918–929.
Ahadian, S., Ramón-Azcón, J., Ostrovidov, S., Camci-Unal, G., Hosseini, V., Kaji, H., Ino, K., Shiku, H., Khademhosseini, A., & Matsue, T. (2012). Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab on a Chip, 12, 3491–3503.
Billiet, T., Vandenhaute, M., Schelfhout, J., Van Vlierberghe, S., & Dubruel, P. (2012). A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials, 33, 6020–6041.
Murtuza, B., Nichol, J., & Khademhosseini, A. (2009). Micro- and nanoscale control of the cardiac stem cell niche for tissue fabrication. Tissue Engineering. Part B, Reviews, 15, 443–454.
Aubin, H., Nichol, J., Hutson, C., Bae, H., Sieminski, A., Cropek, D., et al. (2010). Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials, 31, 6941–6951.
Klotz, B., Gawlitta, D., Rosenberg, A., Malda, J., & Melchels, F. (2016). Gelatin-Methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends in Biotechnology, 34, 394–407.
Celikkin, N., Mastrogiacomo, S., Jaroszewicz, J., Walboomers, X., & Swieszkowski, W. (2018). Gelatin methacrylate scaffold for bone tissue engineering: The influence of polymer concentration. Journal of Biomedical Materials Research. Part A, 106, 201–209.
Schuurman, W., Levett, P., Pot, M., van Weeren, P., Dhert, W., Hutmacher, D., et al. (2013). Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs. Macromolecular Bioscience, 13, 551–561.
Monteiro, N., Thrivikraman, G., Athirasala, A., Tahayeri, A., França, C., Ferracane, J., et al. (2018). Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dental Materials, 34, 389–399.
Shin, H., Nichol, J., & Khademhosseini, A. (2011). Cell-adhesive and mechanically tunable glucose-based biodegradable hydrogels. Acta Biomaterialia, 7, 106–114.
Coutinho, D., Sant, S., Shin, H., Oliveira, J., Gomes, M., Neves, N., et al. (2010). Modified Gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials, 31, 7494–7502.
Assmann, A., Vegh, A., Ghasemi-Rad, M., Bagherifard, S., Cheng, G., Sani, E., et al. (2017). A highly adhesive and naturally derived sealant. Biomaterials, 140, 115–127.
Lee, Y., Lee, J., Bae, P., Chung, I., Chung, B., & Chung, B. (2015). Photo-crosslinkable hydrogel-based 3D microfluidic culture device. Electrophoresis, 36, 994–1001.
Wang, Z., Kumar, H., Tian, Z., Jin, X., Holzman, J., Menard, F., et al. (2018). Visible light Photoinitiation of cell-adhesive gelatin Methacryloyl hydrogels for Stereolithography 3D bioprinting. ACS Applied Materials & Interfaces, 10, 26859–26869.
Bartnikowski, M., Bartnikowski, N., Woodruff, M., Schrobback, K., & Klein, T. (2015). Protective effects of reactive functional groups on chondrocytes in photocrosslinkable hydrogel systems. Acta Biomaterialia, 27, 66–76.
Chen, Y., Lin, R., Qi, H., Yang, Y., Bae, H., Melero-Martin, J., et al. (2012). Functional human vascular network generated in Photocrosslinkable gelatin methacrylate hydrogels. Advanced Functional Materials, 22, 2027–2039.
Lee, B., Lum, N., Seow, L., Lim, P., & Tan, L. (2016). Synthesis and characterization of types a and B gelatin Methacryloyl for bioink applications. Materials (Basel), 9.
Nikkhah, M., Eshak, N., Zorlutuna, P., Annabi, N., Castello, M., Kim, K., Dolatshahi-Pirouz, A., Edalat, F., Bae, H., Yang, Y., & Khademhosseini, A. (2012). Directed endothelial cell morphogenesis in micropatterned gelatin methacrylate hydrogels. Biomaterials, 33, 9009–9018.
Lin, C., Su, J., Lee, S., & Lin, Y. (2018). Stiffness modification of Photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine.
Eke, G., Mangir, N., Hasirci, N., MacNeil, S., & Hasirci, V. (2017). Development of a UV crosslinked biodegradable hydrogel containing adipose derived stem cells to promote vascularization for skin wounds and tissue engineering. Biomaterials, 129, 188–198.
Gao, G., Schilling, A., Hubbell, K., Yonezawa, T., Truong, D., Hong, Y., et al. (2015). Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnology Letters, 37, 2349–2355.
Bae, J., Lee, J., & Chung, B. (2016). Hydrogel-encapsulated 3D microwell array for neuronal differentiation. Biomedical Materials, 11, 015019.
Zheng, J., Zhao, F., Zhang, W., Mo, Y., Zeng, L., Li, X., & Chen, X. (2018). Sequentially-crosslinked biomimetic bioactive glass/gelatin methacryloyl composites hydrogels for bone regeneration. Materials Science & Engineering. C, Materials for Biological Applications, 89, 119–127.
Li, H., Tan, Y., Liu, S., & Li, L. (2018). Three-dimensional bioprinting of oppositely charged hydrogels with super strong Interface bonding. ACS Applied Materials & Interfaces, 10, 11164–11174.
DeForest, C., & Anseth, K. (2012). Advances in bioactive hydrogels to probe and direct cell fate. Annu Rev Chem Biomol Eng, 3, 421–444.
Malda, J., Visser, J., Melchels, F., Jüngst, T., Hennink, W., Dhert, W., et al. (2013). 25th anniversary article: Engineering hydrogels for biofabrication. Adv Mater Weinheim, 25, 5011–5028.
Liu, Y., & Chan-Park, M. (2009). Hydrogel based on interpenetrating polymer networks of dextran and gelatin for vascular tissue engineering. Biomaterials, 30, 196–207.
Suri, S., & Schmidt, C. (2009). Photopatterned collagen-hyaluronic acid interpenetrating polymer network hydrogels. Acta Biomaterialia, 5, 2385–2397.
Ramón-Azcón, J., Ahadian, S., Estili, M., Liang, X., Ostrovidov, S., Kaji, H., Shiku, H., Ramalingam, M., Nakajima, K., Sakka, Y., Khademhosseini, A., & Matsue, T. (2013). Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Adv Mater Weinheim, 25, 4028–4034.
Ahadian, S., Yamada, S., Ramón-Azcón, J., Estili, M., Liang, X., Nakajima, K., Shiku, H., Khademhosseini, A., & Matsue, T. (2016). Hybrid hydrogel-aligned carbon nanotube scaffolds to enhance cardiac differentiation of embryoid bodies. Acta Biomaterialia, 31, 134–143.
Stratesteffen, H., Köpf, M., Kreimendahl, F., Blaeser, A., Jockenhoevel, S., & Fischer, H. (2017). GelMA-collagen blends enable drop-on-demand 3D printablility and promote angiogenesis. Biofabrication, 9, 045002.
Xiao, W., He, J., Nichol, J., Wang, L., Hutson, C., Wang, B., et al. (2011). Synthesis and characterization of photocrosslinkable gelatin and silk fibroin interpenetrating polymer network hydrogels. Acta Biomaterialia, 7, 2384–2393.
Pacelli, S., Rampetsreiter, K., Modaresi, S., Subham, S., Chakravarti, A., Lohfeld, S., et al. (2018). Fabrication of a double-cross-linked interpenetrating polymeric network (IPN) hydrogel surface modified with Polydopamine to modulate the osteogenic differentiation of adipose-derived stem cells. ACS Applied Materials & Interfaces, 10, 24955–24962.
Zuo, Y., Liu, X., Wei, D., Sun, J., Xiao, W., Zhao, H., Guo, L., Wei, Q., Fan, H., & Zhang, X. (2015). Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Applied Materials & Interfaces, 7, 10386–10394.
Suo, H., Xu, K., & Zheng, X. (2015). Using glucosamine to improve the properties of photocrosslinked gelatin scaffolds. Journal of Biomaterials Applications, 29, 977–987.
Soucy, J., Shirzaei Sani, E., Portillo Lara, R., Diaz, D., Dias, F., Weiss, A., et al. (2018). Photocrosslinkable gelatin/Tropoelastin hydrogel adhesives for peripheral nerve repair. Tissue Engineering. Part A, 24, 1393–1405.
Wang, H., Zhou, L., Liao, J., Tan, Y., Ouyang, K., Ning, C., Ni, G., & Tan, G. (2014). Cell-laden photocrosslinked GelMA-DexMA copolymer hydrogels with tunable mechanical properties for tissue engineering. Journal of Materials Science. Materials in Medicine, 25, 2173–2183.
Cha, C., Oh, J., Kim, K., Qiu, Y., Joh, M., Shin, S., et al. (2014). Microfluidics-assisted fabrication of gelatin-silica core-shell microgels for injectable tissue constructs. Biomacromolecules, 15, 283–290.
Daniele, M., Adams, A., Naciri, J., North, S., & Ligler, F. (2014). Interpenetrating networks based on gelatin methacrylamide and PEG formed using concurrent thiol click chemistries for hydrogel tissue engineering scaffolds. Biomaterials, 35, 1845–1856.
Visser, J., Melchels, F., Jeon, J., van Bussel, E., Kimpton, L., Byrne, H., et al. (2015). Reinforcement of hydrogels using three-dimensionally printed microfibres. Nature Communications, 6, 6933.
Luan, C., Wang, H., Han, Q., Ma, X., Zhang, D., Xu, Y., Chen, B., Li, M., & Zhao, Y. (2018). Folic acid-functionalized hybrid photonic barcodes for capture and release of circulating tumor cells. ACS Applied Materials & Interfaces, 10, 21206–21212.
Miri, A., Nieto, D., Iglesias, L., Goodarzi Hosseinabadi, H., Maharjan, S., Ruiz-Esparza, G., et al. (2018). Microfluidics-enabled multimaterial Maskless stereolithographic bioprinting. Adv Mater Weinheim, 30, e1800242.
Shin, S., Bae, H., Cha, J., Mun, J., Chen, Y., Tekin, H., et al. (2012). Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano, 6, 362–372.
Shin, S., Jung, S., Zalabany, M., Kim, K., Zorlutuna, P., Kim, S., et al. (2013). Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano, 7, 2369–2380.
Ahadian, S., Ramón-Azcón, J., Estili, M., Liang, X., Ostrovidov, S., Shiku, H., et al. (2014). Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Scientific Reports, 4, 4271.
Sun, H., Tang, J., Mou, Y., Zhou, J., Qu, L., Duval, K., Huang, Z., Lin, N., Dai, R., Liang, C., Chen, Z., Tang, L., & Tian, F. (2017). Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through β1-integrin mediated FAK and RhoA pathway. Acta Biomaterialia, 48, 88–99.
Paul, A., Hasan, A., Kindi, H., Gaharwar, A., Rao, V., Nikkhah, M., et al. (2014). Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano, 8, 8050–8062.
Cha, C., Shin, S., Gao, X., Annabi, N., Dokmeci, M., Tang, X., et al. (2014). Controlling mechanical properties of cell-laden hydrogels by covalent incorporation of graphene oxide. Small, 10, 514–523.
Shin, S., Zihlmann, C., Akbari, M., Assawes, P., Cheung, L., Zhang, K., et al. (2016). Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small, 12, 3677–3689.
Vashist, S., Zheng, D., Al-Rubeaan, K., Luong, J., & Sheu, F. (2011). Advances in carbon nanotube based electrochemical sensors for bioanalytical applications. Biotechnology Advances, 29, 169–188.
Fabbro, C., Ali-Boucetta, H., Da Ros, T., Kostarelos, K., Bianco, A., & Prato, M. (2012). Targeting carbon nanotubes against cancer. Chem Commun (Camb), 48, 3911–3926.
Shim, J., Grosberg, A., Nawroth, J. C., Parker, K. K., & Bertoldi, K. (2012). Modeling of cardiac muscle thin films: Pre-stretch, passive and active behavior. Journal of Biomechanics, 45, 832–841.
Liu, W., Zhong, Z., Hu, N., Zhou, Y., Maggio, L., Miri, A., et al. (2018). Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments. Biofabrication, 10, 024102.
Levett, P., Melchels, F., Schrobback, K., Hutmacher, D., Malda, J., & Klein, T. (2014). A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomaterialia, 10, 214–223.
Boere, K., Visser, J., Seyednejad, H., Rahimian, S., Gawlitta, D., van Steenbergen, M., et al. (2014). Covalent attachment of a three-dimensionally printed thermoplast to a gelatin hydrogel for mechanically enhanced cartilage constructs. Acta Biomaterialia, 10, 2602–2611.
Gauvin, R., Chen, Y., Lee, J., Soman, P., Zorlutuna, P., Nichol, J., et al. (2012). Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials, 33, 3824–3834.
Wang, J., Li, H., Yao, Y., Zhao, T., Chen, Y., Shen, Y., Wang, L. L., & Zhu, Y. (2018). Stem cell-derived mitochondria transplantation: A novel strategy and the challenges for the treatment of tissue injury. Stem Cell Research & Therapy, 9, 106.
Bianco, P., & Robey, P. (2001). Stem cells in tissue engineering. Nature, 414, 118–121.
Liu, S., Schackel, T., Weidner, N., & Puttagunta, R. (2017). Biomaterial-supported cell transplantation treatments for spinal cord injury: Challenges and perspectives. Frontiers in Cellular Neuroscience, 11, 430.
West, J. (2011). Protein-patterned hydrogels: Customized cell microenvironments. Nature Materials, 10, 727–729.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Yamanaka, S. (2012). Induced pluripotent stem cells: Past, present, and future. Cell Stem Cell, 10, 678–684.
DeBrot, A., & Yao, L. (2018). The combination of induced pluripotent stem cells and bioscaffolds holds promise for spinal cord regeneration. Neural Regeneration Research, 13, 1677–1684.
Fan, L., Liu, C., Chen, X., Zou, Y., Zhou, Z., Lin, C., Tan, G., Zhou, L., Ning, C., & Wang, Q. (2018). Directing induced pluripotent stem cell derived neural stem cell fate with a three-dimensional biomimetic hydrogel for spinal cord injury repair. ACS Applied Materials & Interfaces, 10, 17742–17755.
Cízková, D., Rosocha, J., Vanický, I., Jergová, S., & Cízek, M. (2006). Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cellular and Molecular Neurobiology, 26, 1167–1180.
Shao, N., Guo, J., Guan, Y., Zhang, H., Li, X., Chen, X., Zhou, D., & Huang, Y. (2018). Development of organic/inorganic compatible and sustainably bioactive composites for effective bone regeneration. Biomacromolecules, 19, 3637–3648.
Visser, J., Gawlitta, D., Benders, K., Toma, S., Pouran, B., van Weeren, P., et al. (2015). Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials, 37, 174–182.
Chen, X., Katakowski, M., Li, Y., Lu, D., Wang, L., Zhang, L., Chen, J., Xu, Y., Gautam, S., Mahmood, A., & Chopp, M. (2002). Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. Journal of Neuroscience Research, 69, 687–691.
Mendonça, M., Larocca, T., de Freitas Souza, B., Villarreal, C., Silva, L., Matos, A., et al. (2014). Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Research & Therapy, 5, 126.
Su, P., Tian, Y., Yang, C., Ma, X., Wang, X., Pei, J., & Qian, A. (2018). Mesenchymal stem cell migration during bone formation and bone diseases therapy. International Journal of Molecular Sciences, 19.
Hu, M., Borrelli, M., Lorenz, H., Longaker, M., & Wan, D. (2018). Mesenchymal stromal cells and cutaneous wound healing: A comprehensive review of the background, role, and therapeutic potential. Stem Cells International, 2018, 1–13.
Sheehy, E., Mesallati, T., Kelly, L., Vinardell, T., Buckley, C., & Kelly, D. (2015). Tissue engineering whole bones through endochondral ossification: Regenerating the distal phalanx. Biores Open Access, 4, 229–241.
Mesallati, T., Sheehy, E., Vinardell, T., Buckley, C., & Kelly, D. (2015). Tissue engineering scaled-up, anatomically shaped osteochondral constructs for joint resurfacing. European Cells & Materials, 30, 163–185; discussion 85-6.
Daly, A., Pitacco, P., Nulty, J., Cunniffe, G., & Kelly, D. (2018). 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials, 162, 34–46.
Erkoc, P., Seker, F., Bagci-Onder, T., & Kizilel, S. (2018). Gelatin Methacryloyl hydrogels in the absence of a Crosslinker as 3D glioblastoma Multiforme (GBM)-mimetic microenvironment. Macromolecular Bioscience, 18.
Lin, R., Chen, Y., Moreno-Luna, R., Khademhosseini, A., & Melero-Martin, J. (2013). Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials, 34, 6785–6796.
McKay, R. (1997). Stem cells in the central nervous system. Science, 276, 66–71.
Han, S., Kang, D., Mujtaba, T., Rao, M., & Fischer, I. (2002). Grafted lineage-restricted precursors differentiate exclusively into neurons in the adult spinal cord. Experimental Neurology, 177, 360–375.
Huang, L., & Wang, G. (2017). The effects of different factors on the behavior of neural stem cells. Stem Cells International, 2017, 9497325.
Zhou, X., Cui, H., Nowicki, M., Miao, S., Lee, S. J., Masood, F., Harris, B.T., Zhang, L.G. (2018). Three-dimensional-bioprinted dopamine-based matrix for promoting neural regeneration. ACS Applied Materials & Interfaces, 10(10), 8993-9001.
Gronthos, S., Franklin, D., Leddy, H., Robey, P., Storms, R., & Gimble, J. (2001). Surface protein characterization of human adipose tissue-derived stromal cells. Journal of Cellular Physiology, 189, 54–63.
Wagner, W., Wein, F., Seckinger, A., Frankhauser, M., Wirkner, U., Krause, U., Blake, J., Schwager, C., Eckstein, V., Ansorge, W., & Ho, A. D. (2005). Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Experimental Hematology, 33, 1402–1416.
Fraser, J., Wulur, I., Alfonso, Z., & Hedrick, M. (2006). Fat tissue: An underappreciated source of stem cells for biotechnology. Trends in Biotechnology, 24, 150–154.
Onofrillo, C., Duchi, S., O'Connell, C., Blanchard, R., O'Connor, A., Scott, M., et al. (2018). Biofabrication of human articular cartilage: A path towards the development of a clinical treatment. Biofabrication, 10, 045006.
McKay, R. (2000). Stem cells — Hype and hope. Nature, 406, 361–364.
Ahadian, S., Yamada, S., Ramón-Azcón, J., Ino, K., Shiku, H., Khademhosseini, A., & Matsue, T. (2014). Rapid and high-throughput formation of 3D embryoid bodies in hydrogels using the dielectrophoresis technique. Lab on a Chip, 14, 3690–3694.
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America, 78, 7634–7638.
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292, 154–156.
Park, I. H., Arora, N., Huo, H., Maherali, N., Ahfeldt, T., Shimamura, A., Lensch, M. W., Cowan, C., Hochedlinger, K., & Daley, G. Q. (2008). Disease-specific induced pluripotent stem cells. Cell, 134, 877–886.
Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (Grant Nos. 81401011, 81572229, 81673777), the Natural Science Foundation of Zhejiang, China (Grant No.LY15H060004).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There are no conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, S., Zhao, T., Wang, J. et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: an Effective Strategy for Tissue Engineering. Stem Cell Rev and Rep 15, 664–679 (2019). https://doi.org/10.1007/s12015-019-09893-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09893-4