Abstract
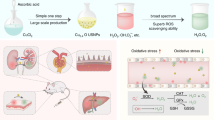
Renal fibrosis is a hallmark feature of chronic kidney diseases (CKDs). However, despite the increased prevalence of renal fibrosis, there is no approved antifibrotic drug for the management of renal fibrosis. Cerium oxide nanoparticles (CONPs) have been demonstrated to possess a number of properties including antioxidant, anti-inflammatory and nephroprotective activity. As the kidneys are rich in lactoferrin (Lf) receptors, we synthesised the lactoferrin-CONP (Lf-CONP) system to be used for active targeting of the kidneys and provide antifibrotic effects of CONPs to the kidneys. We used the unilateral ureteral obstruction (UUO)–induced renal fibrosis model and treated the animals with Lf-CONP to observe the antifibrotic effects of Lf-CONP. Lf-CONP was found to inhibit the progression of renal fibrosis in a superior manner when compared to CONPs alone.




Similar content being viewed by others
Data Availability
Available on request
References
Lv W, Booz GW, Fan F et al (2018) Oxidative stress and renal fibrosis: recent insights for the development of novel therapeutic strategies. Front Physiol 9:105
Su H, Wan C, Song A et al (2019) Oxidative stress and renal fibrosis: mechanisms and therapies. Ren Fibros Mech Ther 1165:585–604
Honda T, Hirakawa Y, Nangaku M (2019) The role of oxidative stress and hypoxia in renal disease. Kidney Res Clin Pract 38:414–426
François H, Chatziantoniou C (2018) Renal fibrosis: recent translational aspects. Matrix Biol 68:318–332
Meng X, Nikolic-Paterson DJ, Lan HY (2016) TGF-β: the master regulator of fibrosis. Nat Rev Nephrol 12:325–338
Najafi R, Hosseini A, Ghaznavi H et al (2017) Neuroprotective effect of cerium oxide nanoparticles in a rat model of experimental diabetic neuropathy. Brain Res Bull 131:117–122
Pourkhalili N, Hosseini A, Nili-Ahmadabadi A et al (2011) Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes 2:204–210
Oró D, Yudina T, Fernández-Varo G et al (2016) Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J Hepatol 64:691–698
Chen J, Patil S, Seal S, McGinnis JF (2006) Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol 1:142–150
Nelson BC, Johnson ME, Walker ML et al (2016) Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 5:15
Nourmohammadi E, Khoshdel-Sarkarizi H, Nedaeinia R et al (2019) Evaluation of anticancer effects of cerium oxide nanoparticles on mouse fibrosarcoma cell line. J Cell Physiol 234:4987–4996
Hirst SM, Karakoti AS, Tyler RD et al (2009) Anti-inflammatory properties of cerium oxide nanoparticles. Small 5:2848–2856
Popov AL, Zaichkina SI, Popova NR et al (2016) Radioprotective effects of ultra-small citrate-stabilized cerium oxide nanoparticles in vitro and in vivo. RSC Adv 6:106141–106149
Saifi MA, Peddakkulappagari CS, Ahmad A, Godugu C (2020) Leveraging the pathophysiological alterations of obstructive nephropathy to treat renal fibrosis by cerium oxide nanoparticles. ACS Biomater Sci Eng 6:3563–3573
Liang X, Wang H, Zhu Y et al (2016) Short-and long-term tracking of anionic ultrasmall nanoparticles in kidney. ACS Nano 10:387–395
Åbrink M, Larsson E, Gobl A, Hellman L (2000) Expression of lactoferrin in the kidney: implications for innate immunity and iron metabolism. Kidney Int 57:2004–2010
Hsu Y-H, Chiu I-J, Lin Y-F et al (2020) Lactoferrin contributes a renoprotective effect in acute kidney injury and early renal fibrosis. Pharmaceutics 12:434
Annaldas S, Saifi MA, Khurana A, Godugu C (2019) Nimbolide ameliorates unilateral ureteral obstruction-induced renal fibrosis by inhibition of TGF-β and EMT/Slug signalling. Mol Immunol 112:247–255
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys acta (BBA)-general Subj 582:67–78
Bansod S, Godugu C (2021) Nimbolide ameliorates pancreatic inflammation and apoptosis by modulating NF-κB/SIRT1 and apoptosis signaling in acute pancreatitis model. Int Immunopharmacol 90:107246
Neuman RE, Logan MA (1950) The determination of hydroxyproline. J Biol Chem 184:299–306
Sravani S, Saifi MA, Godugu C (2020) Riociguat ameliorates kidney injury and fibrosis in an animal model. Biochem Biophys Res Commun 530:706–712
Jester JV, Barry-Lane PA, Petroll WM et al (1997) Inhibition of corneal fibrosis by topical application of blocking antibodies to TGF beta in the rabbit. Cornea 16:177–187
Yanagita M (2012) Inhibitors/antagonists of TGF-β system in kidney fibrosis. Nephrol Dial Transplant 27:3686–3691
Pat B, Yang T, Kong C et al (2005) Activation of ERK in renal fibrosis after unilateral ureteral obstruction: modulation by antioxidants. Kidney Int 67:931–943
Kawai Y, Satoh T, Hibi D et al (2009) The effect of antioxidant on development of fibrosis by cisplatin in rats. J Pharmacol Sci 111:433–439
Chade AR, Rodriguez-Porcel M, Herrmann J et al (2004) Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol 15:958–966
Hirst SM, Karakoti A, Singh S et al (2013) Bio-distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol 28:107–118
Lesná J, Tichá A, Hyšpler R et al (2015) Omentin-1 plasma levels and cholesterol metabolism in obese patients with diabetes mellitus type 1: impact of weight reduction. Nutr Diabetes 5:e183–e183
Grey A, Banovic T, Zhu Q et al (2004) The low-density lipoprotein receptor-related protein 1 is a mitogenic receptor for lactoferrin in osteoblastic cells. Mol Endocrinol 18:2268–2278
Ando K, Hasegawa K, Shindo K et al (2010) Human lactoferrin activates NF-κB through the Toll-like receptor 4 pathway while it interferes with the lipopolysaccharide-stimulated TLR4 signaling. FEBS J 277:2051–2066
Takayama Y, Aoki R, Uchida R et al (2017) Role of CXC chemokine receptor type 4 as a lactoferrin receptor. Biochem Cell Biol 95:57–63
Penco S, Caligo MA, Cipollini G et al (1999) Lactoferrin expression in human breast cancer. Cancer Biochem Biophys 17:163–178
Benaïssa M, Peyrat J, Hornez L et al (2005) Expression and prognostic value of lactoferrin mRNA isoforms in human breast cancer. Int J cancer 114:299–306
Abdelmoneem MA, Mahmoud M, Zaky A et al (2018) Decorating protein nanospheres with lactoferrin enhances oral COX-2 inhibitor/herbal therapy of hepatocellular carcinoma. Nanomedicine 13:2377–2395
Hakim F, Wang Y, Zhang SXL et al (2014) Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res 74:1329–1337
Bezault J, Bhimani R, Wiprovnick J, Furmanski P (1994) Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res 54:2310–2312
Lord MS, Farrugia BL, Yan CMY et al (2016) Hyaluronan coated cerium oxide nanoparticles modulate CD44 and reactive oxygen species expression in human fibroblasts. J Biomed Mater Res Part A 104:1736–1746
Hijaz M, Das S, Mert I et al (2016) Folic acid tagged nanoceria as a novel therapeutic agent in ovarian cancer. BMC Cancer 16:1–14
Zhao H, Liu Y, Liu Z et al (2017) Role of mitochondrial dysfunction in renal fibrosis promoted by hypochlorite-modified albumin in a remnant kidney model and protective effects of antioxidant peptide SS-31. Eur J Pharmacol 804:57–67
Liu H, Wu H, Zhu N et al (2020) Lactoferrin protects against iron dysregulation, oxidative stress, and apoptosis in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced Parkinson’s disease in mice. J Neurochem 152:397–415
Safaeian L, Javanmard SH, Mollanoori Y, Dana N (2015) Cytoprotective and antioxidant effects of human lactoferrin against H2O2-induced oxidative stress in human umbilical vein endothelial cells. Adv Biomed Res 4:188
Han N, Li H, Li G et al (2019) Effect of bovine lactoferrin as a novel therapeutic agent in a rat model of sepsis-induced acute lung injury. AMB Express 9:1–8
Hegazy R, Salama A, Mansour D, Hassan A (2016) Renoprotective effect of lactoferrin against chromium-induced acute kidney injury in rats: involvement of IL-18 and IGF-1 inhibition. PLoS ONE 11:e0151486
Akhtar MJ, Ahamed M, Alhadlaq HA et al (2015) Glutathione replenishing potential of CeO2 nanoparticles in human breast and fibrosarcoma cells. J Colloid Interface Sci 453:21–27
Kumari P, Saifi MA, Khurana A, Godugu C (2018) Cardioprotective effects of nanoceria in a murine model of cardiac remodeling. J Trace Elem Med Biol 50:198–208
Wallach TE, Srivastava V, Reyes E et al (2019) Lactoferrin reverses methotrexate driven epithelial barrier defect by inhibiting TGF-β mediated epithelial to mesenchymal transition. bioRxiv
Guo C, Smith R, Gant TW, Leonard MO (2015) Cerium dioxide nanoparticles protect against oxidative stress induced injury through modulation of TGF-β signalling. Toxicol Res (Camb) 4:464–475
Domala A, Bale S, Godugu C (2020) Protective effects of nanoceria in imiquimod induced psoriasis by inhibiting the inflammatory responses. Nanomedicine 15:5–22
Acknowledgements
The authors would also like to acknowledge Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Government of India and the Director, NIPER-Hyderabad.
Funding
The authors would like to thank the Department of Biotechnology (DBT), Govt. of India, for the financial support via North East-Twinning Grant to Dr. CG (MAP/2015/58), DBT Indo-Brazil Grant DBT/IC-2/Indo-Brazil/2016–19/01, and the Department of Science and Technology-Science and Engineering Board-Early Career Research Award (SERB-ECR) Grant ECR/2016/000007.
Author information
Authors and Affiliations
Contributions
MAS performed the experiments, collected the data, analysed the results and prepared the draft of the manuscript. RH helped in analysing the results and contributed to the writing of the manuscript. CG designed the experiment, provided the facilities, supervised the work, edited the manuscript draft and approved the publication of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experiments were conducted after obtaining approval from the Institutional Animal Ethics Committee (IAEC, NIP/01/2018/RT/277). All the experiments were performed in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aslam Saifi, M., Hirawat, R. & Godugu, C. Lactoferrin-Decorated Cerium Oxide Nanoparticles Prevent Renal Injury and Fibrosis. Biol Trace Elem Res 201, 1837–1845 (2023). https://doi.org/10.1007/s12011-022-03284-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03284-6