Abstract
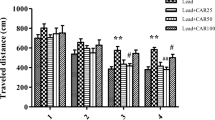
Developmental lead (Pb) exposure involves various serious consequences, especially leading to neurotoxicity. In this study, we examined the possible role of monosialoganglioside (GM1) in lead-induced nervous impairment in the developing rat. Newborn male Sprague-Dawley rat pups were exposed to lead from birth for 30 days and then subjected to GM1 administration (0.4, 2, or 10 mg/kg; i.p.) or 0.9% saline. The results showed that developmental lead exposure significantly impaired spatial learning and memory in the Morris water maze test, reduced GM1 content, induced oxidative stress, and weakened the antioxidative systems in the hippocampus. However, co-treatment with GM1 reversed these effects. Moreover, GM1 counteracted lead-induced apoptosis by decreasing the expression of Bax, cleaved caspase-3, and by increasing the level of Bcl-2 in a dose-dependent manner. Furthermore, we found that GM1 upregulated the expression of SIRT1, CREB phosphorylation, and BDNF, which underlie learning and memory in the lead-treated developing rat hippocampus. In conclusion, our study demonstrated that GM1 exerts a protective effect on lead-induced cognitive deficits via antioxidant activity, preventing apoptosis, and activating SIRT1/CREB/BDNF in the developing rat hippocampus, implying a novel potential assistant therapy for lead poisoning.







Similar content being viewed by others
References
Health CE (2016) Prevention of childhood lead toxicity. Pediatrics 138:e20161493–e20161493. https://doi.org/10.1542/peds.2016-1493
Abdou HM, Hassan MA (2014) Protective role of omega-3 polyunsaturated fatty acid against lead acetate-induced toxicity in liver and kidney of female rats. Biomed Res Int 2014:435857. https://doi.org/10.1155/2014/435857
Boskabady MH, Tabatabai SA, Farkhondeh T (2016) Inhaled lead affects lung pathology and inflammation in sensitized and control Guinea pigs. Environ Toxicol 31:452–460. https://doi.org/10.1002/tox.22058
Luo W, Ruan D, Yan C, Yin S, Chen J (2012) Effects of chronic lead exposure on functions of nervous system in Chinese children and developmental rats. Neurotoxicology 33:862–871. https://doi.org/10.1016/j.neuro.2012.03.008
Mabrouk A, Ben Cheikh H (2016) Thymoquinone supplementation ameliorates lead-induced testis function impairment in adult rats. Toxicol Ind Health 32:1114–1121. https://doi.org/10.1177/0748233714548474
Senut M-C, Cingolani P, Sen A, Kruger A, Shaik A, Hirsch H, Suhr ST, Ruden D (2012) Epigenetics of early-life lead exposure and effects on brain development. Epigenomics 4:665–674. https://doi.org/10.2217/epi.12.58
Sharifi AM, Ghazanfari R, Tekiyehmaroof N, Sharifi MA (2011) Investigating the effect of lead acetate on rat bone marrow-derived mesenchymal stem cells toxicity: role of apoptosis. Toxicol Mech Methods 21:225–230. https://doi.org/10.3109/15376516.2010.543943
Canfield RL, Henderson CRJ, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP (2003) Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 348:1517–1526. https://doi.org/10.1056/NEJMoa022848
Skerfving S, Lofmark L, Lundh T, Mikoczy Z, Stromberg U (2015) Late effects of low blood lead concentrations in children on school performance and cognitive functions. Neurotoxicology 49:114–120. https://doi.org/10.1016/j.neuro.2015.05.009
Wu J, Basha MR, Brock B, Cox DP, Cardozo-Pelaez F, McPherson CA, Harry J, Rice DC, Maloney B, Chen D, Lahiri DK, Zawia NH (2008) Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci 28:3–9. https://doi.org/10.1523/JNEUROSCI.4405-07.2008
Zhou C-C, Gao Z-Y, Wang J, Wu M-Q, Hu S, Chen F, Liu J-X, Pan H, Yan C-H (2018) Lead exposure induces Alzheimers's disease (AD)-like pathology and disturbes cholesterol metabolism in the young rat brain. Toxicol Lett 296:173–183. https://doi.org/10.1016/j.toxlet.2018.06.1065
Horio Y, Hayashi T, Kuno A, Kunimoto R (2011) Cellular and molecular effects of sirtuins in health and disease. Clin Sci (Lond) 121(5):191–203. https://doi.org/10.1042/cs20100587
Hori YS, Kuno A, Hosoda R, Horio Y (2013) Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One 8(9):e73875. https://doi.org/10.1371/journal.pone.0073875
Gao J, Wang W-Y, Mao Y-W, Graff J, Guan J-S, Pan L, Mak G, Kim D, Su SC, Tsai L-H (2010) A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466:1105–1109. https://doi.org/10.1038/nature09271
Kida S, Serita T (2014) Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull 105:17–24. https://doi.org/10.1016/j.brainresbull.2014.04.011
Alberini CM, Kandel ER (2014) The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol 7:a021741. https://doi.org/10.1101/cshperspect.a021741
Ledeen RW (1978) Ganglioside structures and distribution: are they localized at the nerve ending? J Supramol Struct 8:1–17. https://doi.org/10.1002/jss.400080102
Ba X-h (2016) Therapeutic effects of GM1 on Parkinson's disease in rats and its mechanism. Int J Neurosci 126:163–167. https://doi.org/10.3109/00207454.2014.996640
Di Pardo A, Maglione V, Alpaugh M, Horkey M, Atwal RS, Sassone J, Ciammola A, Steffan JS, Fouad K, Truant R, Sipione S (2012) Ganglioside GM1 induces phosphorylation of mutant huntingtin and restores normal motor behavior in Huntington disease mice. Proc Natl Acad Sci U S A 109:3528–3533. https://doi.org/10.1073/pnas.1114502109
Kreutz F, Frozza RL, Breier AC, De Oliveira VA, Horn AP, Pettenuzzo LF, Netto CA, Salbego CG, Trindade VMT (2011) Amyloid-β induced toxicity involves ganglioside expression and is sensitive to GM1 neuroprotective action. Neurochem Int 59:648–655. https://doi.org/10.1016/j.neuint.2011.06.007
Li L, Tian J, Long MK-W, Chen Y, Lu J, Zhou C, Wang T (2016) Protection against experimental stroke by ganglioside GM1 is associated with the inhibition of autophagy. PLoS One 11:e0144219. https://doi.org/10.1371/journal.pone.0144219
Zhu X-Y, Ye M-Y, Zhang A-M, Wang W-D, Zeng F, Li J-L, Fang F (2015) Influence of one-year neurologic outcome of treatment on newborns with moderate and severe hypoxic-ischemic encephalopathy by rhuEP0 combined with ganglioside (GM1). Eur Rev Med Pharmacol Sci 19:3955–3960
Vlasova IA, Zakharova IO, Sokolova TV, Avrova NF (2013) Metabolic effects of ganglioside GM1 on PC12 cells at oxidative stress depend on modulation of activity of tyrosine kinase of trk receptor. Zh Evol Biokhim Fiziol 49:15–23
Gorria M, Huc L, Sergent O, Rebillard A, Gaboriau F, Dimanche-Boitrel M-T, Lagadic-Gossmann D (2006) Protective effect of monosialoganglioside GM1 against chemically induced apoptosis through targeting of mitochondrial function and iron transport. Biochem Pharmacol 72:1343–1353. https://doi.org/10.1016/j.bcp.2006.07.014
Mutoh T, Tokuda A, Miyadai T, Hamaguchi M, Fujiki N (1995) Ganglioside GM1 binds to the Trk protein and regulates receptor function. Proc Natl Acad Sci U S A 92:5087–5091
Rabin SJ, Bachis A, Mocchetti I (2002) Gangliosides activate Trk receptors by inducing the release of neurotrophins. J Biol Chem 277:49466–49472. https://doi.org/10.1074/jbc.M203240200
Zakharova IO, Sokolova TV, Vlasova YA, Furaev VV, Rychkova MP, Avrova NF (2014) GM1 ganglioside activates ERK1/2 and Akt downstream of Trk tyrosine kinase and protects PC12 cells against hydrogen peroxide toxicity. Neurochem Res 39:2262–2275. https://doi.org/10.1007/s11064-014-1428-6
She J-Q, Wang M, Zhu D-M, Sun L-G, Ruan D-Y (2005) Effect of ganglioside on synaptic plasticity of hippocampus in lead-exposed rats in vivo. Brain Res 1060:162–169. https://doi.org/10.1016/j.brainres.2005.08.044
Deniz BF, Confortim HD, Deckmann I, Miguel PM, Bronauth L, de Oliveira BC, Barbosa S, Cechinel LR, Siqueira IR, Pereira LO (2018) Folic acid supplementation during pregnancy prevents cognitive impairments and BDNF imbalance in the hippocampus of the offspring after neonatal hypoxia-ischemia. J Nutr Biochem 60:35–46. https://doi.org/10.1016/j.jnutbio.2018.06.008
Wang J, Gao Z-Y, Yan J, Ying X-L, Tong S-L, Yan C-H (2017) Sex differences in the effects of prenatal lead exposure on birth outcomes. Environ Pollut 225:193–200. https://doi.org/10.1016/j.envpol.2017.03.031
Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y, Chen Q, Hao Q, Bao D, Hou D (2017) Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep 7(1):10114. https://doi.org/10.1038/s41598-017-10693-4
Chen L, Liu P, Feng X, Ma C (2017) Salidroside suppressing LPS-induced myocardial injury by inhibiting ROS-mediated PI3K/Akt/mTOR pathway in vitro and in vivo. J Cell Mol Med 21(12):3178–3189. https://doi.org/10.1111/jcmm.12871
Zhang L, Tu R, Wang Y, Hu Y, Li X, Cheng X, Yin Y, Li W, Huang H (2017) Early-life exposure to lead induces cognitive impairment in elder mice targeting SIRT1 phosphorylation and oxidative alterations. Front Physiol 8
Meng H, Wang L, He J, Wang Z (2016) The protective effect of gangliosides on lead (Pb)-induced neurotoxicity is mediated by autophagic pathways. Int J Environ Res Public Health 13:365. https://doi.org/10.3390/ijerph13040365
Hossain S, Bhowmick S, Jahan S, Rozario L, Sarkar M, Islam S, Basunia MA, Rahman A, Choudhury BK, Shahjalal H (2016) Maternal lead exposure decreases the levels of brain development and cognition-related proteins with concomitant upsurges of oxidative stress, inflammatory response and apoptosis in the offspring rats. Neurotoxicology 56:150–158. https://doi.org/10.1016/j.neuro.2016.07.013
Barkur RR, Bairy LK (2015) Assessment of oxidative stress in hippocampus, cerebellum and frontal cortex in rat pups exposed to lead (Pb) during specific periods of initial brain development. Biol Trace Elem Res 164:212–218. https://doi.org/10.1007/s12011-014-0221-3
Yang R, Wang Q, Min L, Sui R, Li J, Liu X (2013) Monosialoanglioside improves memory deficits and relieves oxidative stress in the hippocampus of rat model of Alzheimer's disease. Neurol Sci 34:1447–1451. https://doi.org/10.1007/s10072-012-1263-y
Gong G, Yin L, Yuan L, Sui D, Sun Y, Fu H, Chen L, Wang X (2018) Ganglioside GM1 protects against high altitude cerebral edema in rats by suppressing the oxidative stress and inflammatory response via the PI3K/AKT-Nrf2 pathway. Mol Immunol 95:91–98. https://doi.org/10.1016/j.molimm.2018.02.001
Renault TT, Manon S (2011) Bax: addressed to kill. Biochimie 93:1379–1391. https://doi.org/10.1016/j.biochi.2011.05.013
Volkmann N, Marassi FM, Newmeyer DD, Hanein D (2014) The rheostat in the membrane: BCL-2 family proteins and apoptosis. Cell Death Differ 21:206–215. https://doi.org/10.1038/cdd.2013.153
Dribben WH, Creeley CE, Farber N (2011) Low-level lead exposure triggers neuronal apoptosis in the developing mouse brain. Neurotoxicol Teratol 33:473–480. https://doi.org/10.1016/j.ntt.2011.05.006
Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK (2003) Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem 85:1026–1036
Chang H-C, Guarente L (2014) SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 25:138–145. https://doi.org/10.1016/j.tem.2013.12.001
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352. https://doi.org/10.1002/emmm.201302451
Hwang J-w, Yao H, Caito S, Sundar IK, Rahman I (2013) Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic Biol Med 61:95–110. https://doi.org/10.1016/j.freeradbiomed.2013.03.015
Michan S, Li Y, Chou MM-H, Parrella E, Ge H, Long JM, Allard JS, Lewis K, Miller M, Xu W, Mervis RF, Chen J, Guerin KI, Smith LEH, McBurney MW, Sinclair DA, Baudry M, de Cabo R, Longo VD (2010) SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci 30:9695–9707. https://doi.org/10.1523/JNEUROSCI.0027-10.2010
Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J (2011) Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res 89:1723–1736. https://doi.org/10.1002/jnr.22725
Feng C, Gu J, Zhou F, Li J, Zhu G, Guan L, Liu H, Du G, Feng J, Liu D, Zhang S, Fan G (2016) The effect of lead exposure on expression of SIRT1 in the rat hippocampus. Environ Toxicol Pharmacol 44:84–92. https://doi.org/10.1016/j.etap.2016.04.008
Acknowledgements
Fei Chen especially wants to thank the support from his senior CanCan Zhou.
Funding
This work was supported by the National Basic Research Program of China (973 Program, 2012CB525001), the National Natural Science Foundation of China (Grant No. 81472993), and National Key R&D Program of China (2017YFC1600500).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was carried out in strict accordance with the international standards of animal care guidelines, and the experiments were approved by Institutional Animal Care Committee at Shanghai Jiao tong University School of Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, F., Zhou, CC., Yang, Y. et al. GM1 Ameliorates Lead-Induced Cognitive Deficits and Brain Damage Through Activating the SIRT1/CREB/BDNF Pathway in the Developing Male Rat Hippocampus. Biol Trace Elem Res 190, 425–436 (2019). https://doi.org/10.1007/s12011-018-1569-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-018-1569-6