Abstract
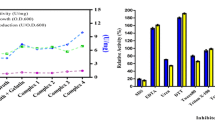
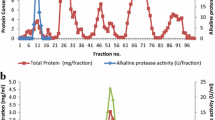
Our newly discovered metalloprotease, designated as ALP NS12 was selected using gelatin agar plates with incubation at 100 °C. Subcloning of the fragments in to pUC118 to make E. coli HB101 (pPEMP01NS) with following two-step chromatography using diethylaminoethyl sepharose (DEAE-sepharose) and Sephadex G-100 columns to purify 97-kDa expressed enzyme was performed. Although activity of immobilized ALP NS12 on glass surface was established at temperatures between 70 and 120 °C and pH ranges 4.0–13.0, the optimum temperature and pH were achieved at 100 °C and 11.0, respectively. Enhancement of enzyme activity was obtained in the presence of 5 mM MnCl2 (91 %), CaCl2 (357 %), FeCl2 (175 %), MgCl2 (94 %), ZnCl2 (412 %), NiCl (86 %), NaCl (239 %), and Na-sulfate (81 %) while inhibition was observed with EDTA (5 mM), PMSF (3 mM), urea (8 M), and SDS (1 %) at 65, 37, 33, and 42 %, respectively. Consequently, the enzyme was well analyzed using crystallography and protein modeling. ALP NS12 can be applied in industrial processes at extreme temperatures and under highly basic conditions, chelators, and detergents.










Similar content being viewed by others
References
Anwar, A., & Saleemuddin, M. (1998). Bioresource Technology, 64, 175–183.
Burhan, A., Nisa, U., Gökhan, C., Ömer, C., Ashabil, A., & Osman, G. (2003). Process Biochemistry, 38, 1397–1403.
Horikoshi, K. (1999). Microbiology and Molecular Biology Reviews, 63, 735–750.
Nima, S., Kambiz, A. N., Zahra, G., Hossien, S. Z., Gholamreza, A., Hakimeh, S., Reza, B., & Hojatollah, V. (2012). Process Biochemistry, 47, 1381–1387.
Nima, S., Padma, R. M. R., & Masoumeh, A. (2012). Starch/Starke, 64, 136–144.
Kanekar, P. P., Nilegaonkar, S. S., Sarnaik, S. S., & Kelkar, A. S. (2002). Bioresource Technology, 85, 87–93.
Setyorini, E., Kim, Y.-J., Takenaka, S., Murakami, S., & Aoki, K. (2006). Journal of Basic Microbiology, 46, 294–304.
Kumari, D., Sharma, N., Pandove, G., & Achal, V. (2009). Life Science Journal, 6, 8–10.
Gupta, M. N., Jain, S., & Roy, I. (2002). Biotechnology Progress, 18, 78–81.
Najafi, M. F., Deobagkar, D., & Deobagkar, D. (2005). Electronic Journal of Biotechnology, 8, 79–85.
Genckal, H., & Tari, C. (2006). Enzyme and Microbial Technology, 39, 703–710.
Silva, C. J. S. M., Zhang, Q., Shen, J., & Cavaco-Paulo, A. (2006). Enzyme and Microbial Technology, 39, 634–640.
Rao, M. B., Tanksale, A. M., Ghatge, M. S., & Deshpande, V. V. (1998). Microbiology and Molecular Biology Reviews, 62, 597–635.
Ravikumar, B., Vacher, C., Berger, Z., Davies, J. E., Luo, S., Oroz, L. G., Scaravilli, F., Easton, D. F., Duden, R., O'Kane, C. J., & Rubinsztein, D. C. (2004). Nature Genetics, 36, 585–595.
Bhaskar, N., Sudeepa, E. S., Rashmi, H. N., & Tamil Selvi, A. (2007). Bioresource Technology, 98, 2758–2764.
Doddapaneni, K. K., Tatineni, R., Vellanki, R. N., Gandu, B., Panyala, N. R., Chakali, B., & Mangamoori, L. N. (2007). Process Biochemistry, 42, 1229–1236.
Padmapriya, B., Rajeswari, T., Nandita, R., & Raj, F. (2012). European Journal of Applied Sciences, 4, 21–26.
Haddar, A., Agrebi, R., Bougatef, A., Hmidet, N., Sellami-Kamoun, A., & Nasri, M. (2009). Bioresource Technology, 100, 3366–3373.
Sellami-Kamoun, A., Haddar, A., Ali, N. E.-H., Ghorbel-Frikha, B., Kanoun, S., & Nasri, M. (2008). Microbiological Research, 163, 299–306.
Adinarayana, K., Ellaiah, P., & Prasad, D. (2003). AAPS PharmSci Tech, 4, 440–448.
Kumar, C. G. (2002). Letters in Applied Microbiology, 34, 13–17.
Garrity, G.M., Bell, J.A., & Lilburn, T.G. (2004). Taxonomic outline of prokaryotes Bergey’s manual of systematic bacteriology, second edition.
Coon, J. J. (2009). Analytical Chemistry, 81, 3208–3215.
Egas, M. C. V., da Costa, M. S., Cowan, D. A., & Pires, E. M. V. (1998). Extremophiles, 2, 23–32.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Journal of Molecular Biology, 215, 403–410.
Kalpana Devi, M., Rasheedha Banu, A., Gnanaprabha, G. R., Pradeep, B. V., & Palaniswamy, M. (2008). Indian Journal of Science and Technology, 7, 1–6.
Saitou, N., & Nei, M. (1987). Molecular Biology and Evolution, 4, 406–425.
Liu, Y. H., Lu, F. P., Li, Y., Yin, X. B., Wang, Y., & Gao, C. (2008). Appl Microbiology and Biotechnology, 78, 85–94.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Journal of Biological Chemistry, 193, 265–275.
Russell, A. J., Drevon, G. F., Wicks, D. & Danielmeier, K. (2005). US Patents 6905733 B2
Nakanishi, T., Matsumura, Y., Minamura, N., & Yamamoto, T. (1974). Agricultural and Biological Chemistry, 38, 37–44.
Feller, G., Narinx, E., Arpigny, J. L., Aittaleb, M., Baise, E., Genicot, S., & Gerday, C. (1996). FEMS Microbiology Reviews, 18, 189–202.
Lo, H. F., Lin, L. L., Chen, H. L., Hsu, W. H., & Chang, C. T. (2001). Process Biochemistry, 36, 743–750.
Ehrenreich, H., Hasselblatt, M., Dembowski, C., Cepek, L., Lewczuk, P., Stiefel, M., Rustenbeck, H., Breiter, N., Jacob, S., Knerlich, F., Bohn, M., Poser, W., Rüther, E., Kochen, M., Gefeller, O., Gleiter, C., Wessel, T. C., Ryck, M. D., Itri, L., Pranged, H., Cerami, A., Brines, M., & Siren, A. L. (2002). Molecular Medicine, 8, 495–505.
Wang, B. C. (1985). Methods in Enzymology, 115, 90–112.
Youssef, N., Sheik, C. S., Krumholz, L. R., Najar, F. Z., Roe, B. A., & Elshahed, M. S. (2009). Applied and Environmental Microbiology, 75, 5227–5236.
Turk, D. (1992). Ph.D. Thesis, TechnischeUniversitatMunchen
Jones, T. A., Zou, J.-Y., Cowan, S. W., & Kjeldgaard, M. (1991). Acta Crystallographica Section A, 47, 110–119.
Tanksale, A., Chandra, P. M., Rao, M., & Deshpande, V. (2001). Biotechnology Letters, 23, 51–54.
Jones, T. A., & Thirup, S. (1986). EMBO J, 5, 819–822.
Brunger, A. T., Kuriyan, J., & Karplus, M. (1987). Science, 235, 458–460.
Deisenhofer, J., Epp, O., Miki, K., Huber, R., & Michel, H. (1985). Nature, 318, 618–624.
Horlein, A. J., Naar, A. M., Heinzel, T., Torchia, J., Gloss, B., Kurokawa, R., Ryan, A., Kamei, Y., Soderstrom, M., Glass, C. K., & Rosenfeld, M. G. (1995). Nature, 377, 397–404.
Tigue, M. A. M., Kelly, C. T., Doyle, E. M., & Fogarty, W. M. (1995). Enzyme and Microbial Technology, 17, 570–573.
Ankaralingam, S., Shankar, T., Ramasubburayan, R., Prakash, S., & Kumar, C. (2012). American-Eurasian Journal of Agriculture and Environmental Science, 2, 1507–1513.
Gupta, A., Roy, I., Patel, R. K., Singh, S. P., Khare, S. K., & Gupta, M. N. (2005). Journal of Chromatography A, 1075, 103–108.
Rahman, R. N. Z. R. A., Geok, L. P., Basri, M., & Salleh, A. B. (2006). Enzyme and Microbial Technology, 39, 1484–1491.
Patil, U., & Chaudhari, A. (2011). Indian Journal of Biotechnology, 10, 329–339.
Asoodeh, A., Mohammadian, H., & Musaabadi, A. (2012). Iranian Journal of Biotechnology, 10, 23–28.
Smith, M., & Zahnley, J. (2005). Journal of Industrial Microbiology and Biotechnology, 32, 277–283.
Acknowledgments
With special thanks to Nooshin Mohebali, Alex Thompson and Zahra Ghasemzadeh who helped us a lot in current research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samie, N., Haerian, B., Muniandy, S. et al. Exhaustive Study of the Novel Hyper Alkalophil, Thermostable, and Chelator Resistant Metalloprotease. Appl Biochem Biotechnol 175, 3397–3417 (2015). https://doi.org/10.1007/s12010-015-1513-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1513-6