Abstract
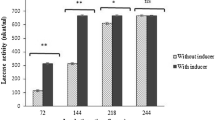
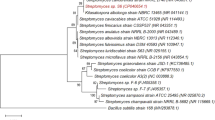
Marine-derived fungi are prone to produce structurally unique secondary metabolites, a considerable number of which display the promising biological properties and/or industrial applications. Among those, ligninolytic enzymes have attracted great interest in recent years. In this work, about 20 strains were isolated from sea mud samples collected in the East China Sea and then screened for their capacity to produce lignin-degrading enzymes. The results showed that a strain, named J63, had a great potential to secrete a considerable amount of laccase. Using molecular method, it was identified as an endophytic fungus, Pestalotiopsis sp. which was rarely reported as ligninolytic enzyme producer in the literature. The production of laccase by Pestalotiopsis sp. J63 was investigated under submerged fermentation (SF) and solid state fermentation (SSF) with various lignocellulosic by-products as substrates. The SSF of rice straw powder accumulated the highest level of laccase activity (10,700 IU/g substrate), whereas the SF of untreated sugarcane bagasse provided the maximum amount of laccase activity (2,000 IU/ml). The value was far higher than those reported by other reports. In addition, it produced 0.11 U/ml cellulase when alkaline-pretreated sugarcane bagasse was used as growth substrate under SF. Meanwhile, the growth of fungi and laccase production under different salinity conditions were also studied. It appeared to be a moderately halo-tolerant organism.









Similar content being viewed by others
References
Reddy, C. A. (1995). The potential for white-rot fungi in the treatment of pollutants. Current Opinion in Biotechnology, 6, 320–328.
Raghukumar, C., D'Souza, T. M., Thorn, R. G., & Reddy, C. A. (1999). Lignin-modifying enzymes of Flavodon flavus, a basidiomycete isolated from a coastal marine environment. Applied and Environmental Microbiology, 65, 2103–2111.
D'Souza, D. T., Tiwari, R., Sah, A. K., & Raghukumar, C. (2006). Enhanced production of laccase by a marine fungus during treatment of colored effluents and synthetic dyes. Enzyme and Microbial Technology, 38, 504–511.
Maciel, M. J. M., Silva, A. C. E., & Ribeiro, H. C. T. (2010). Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota: a review. Electronic Journal of Biotechnology, 13, 6.
Rodriguez Couto, S., & Toca Herrera, J. L. (2006). Industrial and biotechnological applications of laccases: a review. Biotechnology Advances, 24, 500–513.
Pointing, S. B. (2001). Feasibility of bioremediation by white-rot fungi. Applied Microbiology and Biotechnology, 57, 20–33.
Wesenberg, D., Kyriakides, I., & Agathos, S. N. (2003). White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnology Advances, 22, 161–187.
Saparrat, M. C. N., Guillen, F., Arambarri, A. M., Martinez, A. T., & Martinez, M. J. (2002). Induction, isolation, and characterization of two laccases from the white rot basidiomycete Coriolopsis rigida. Applied and Environmental Microbiology, 68, 1534–1540.
Arun, A., Raja, P., Arthi, R., Ananthi, M., Kumar, K., & Eyini, M. (2008). Polycyclic aromatic hydrocarbons (PAHs) Biodegradation by basidiomycetes fungi, Pseudomonas isolate, and their cocultures: comparative in vivo and in silico approach. Applied Biochemistry and Biotechnology, 151, 132–142.
Bugni, T. S., & Ireland, C. M. (2004). Marine-derived fungi: a chemically and biologically diverse group of microorganisms. Natural Product Reports, 21, 143–163.
Jones, E. B. G., Stanley, S. J., & Pinruan, U. (2008). Marine endophyte sources of new chemical natural products: a review. Botanica Marina, 51, 163–170.
Sette, L. D., Passarini, M. R. Z., Rodrigues, M. V. N., & da Silva, M. (2011). Marine-derived filamentous fungi and their potential application for polycyclic aromatic hydrocarbon bioremediation. Marine Pollution Bulletin, 62, 364–370.
Sette, L. D., Bonugli-Santos, R. C., Durrant, L. R., & da Silva, M. (2010). Production of laccase, manganese peroxidase and lignin peroxidase by Brazilian marine-derived fungi. Enzyme and Microbial Technology, 46, 32–37.
Ali, M. S., Saleem, M., Hussain, S., Jabbar, A., Ashraf, M., & Lee, Y. S. (2007). Marine natural products of fungal origin. Natural Product Reports, 24, 1142–1152.
Ebel, R., & Rateb, M. E. (2011). Secondary metabolites of fungi from marine habitats. Natural Product Reports, 28, 290–344.
Newell, S. Y. (1996). Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. Journal of Experimental Marine Biology ad Ecolology, 200, 187–206.n
Elisashvili, V., Kachlishvili, E., Tsiklauri, N., Metreveli, E., Khardziani, T., & Agathos, S. (2009). Lignocellulose-degrading enzyme production by white-rot Basidiomycetes isolated from the forests of Georgia. World Journal of Microbiology & Biotechnology, 25, 331–339.
Mishra, A., & Kumar, S. (2009). Kinetic studies of laccase enzyme of Coriolus versicolor MTCC 138 in an inexpensive culture medium. Biochemical Engineering Journal, 46, 252–256.
Mishra, A., Kumar, S., & Kumar Pandey, A. (2011). Laccase production and simultaneous decolorization of synthetic dyes in unique inexpensive medium by new isolates of white rot fungus. International Biodeterioration and Biodegration, 65, 487–493.
Zeng, X., Cai, Y., Liao, X., Li, W., & Zhang, D. (2011). Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agro-industrial residue. Journal of Hazardour Materials, 187, 517–525.
Rosales, E., Rodríguez Couto, S., & Sanromán, M. A. (2007). Increased laccase production by Trametes hirsuta grown on ground orange peelings. Enzyme and Microbial Technology, 40, 1286–1290.
Elisashvili, V., Penninckx, M., Kachlishvili, E., Tsiklauri, N., Metreveli, E., Kharziani, T., et al. (2008). Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresource Technology, 99, 457–462.
Elisashvili, V., Kachlishvili, E., & Penninckx, M. (2008). Effect of growth substrate, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. Journal of Industrial Microbiology and Biotechnology, 35, 1531–1538.
Levin, L., Herrmann, C., & Papinutti, V. L. (2008). Optimization of lignocellulolytic enzyme production by the white-rot fungus Trametes trogii in solid-state fermentation using response surface methodology. Biochemical Engineering Journal, 39, 207–214.
Gómez, J., Pazos, M., RodrIguez Couto, S., & Sanromán, M. Á. (2005). Chestnut shell and barley bran as potential substrates for laccase production by Coriolopsis rigida under solid-state conditions. Journal of Food Engineering, 68, 315–319.
Couto, S. R., & Toca-Herrera, J. L. (2006). Laccase production at reactor scale by filamentous fungi. Biotechnology Advances, 25, 558–569.
Pandey, A. (1992). Recent process—Developments in solid-state fermentation. Process Biochemistry, 27, 109–117.
Karanth, N. G., Raghavarao, K. S. M. S., & Ranganathan, T. V. (2003). Some engineering aspects of solid-state fermentation. Biochemical Engineering Journal, 13, 127–135.
D’Souza-Ticlo, D., Sharma, D., & Raghukumar, C. (2009). A thermostable metal-tolerant laccase with bioremediation potential from a marine-derived fungus. Marine Biotechnology, 11, 725–737.
Bonugli-Santos, R. C., Durrant, L. R., & Sette, L. D. (2010). Laccase activity and putative laccase genes in marine-derived basidiomycetes. Fungal Biology UK, 114, 863–872.
Baldrian, P. (2003). Interactions of heavy metals with white-rot fungi. Enzyme and Microbial Technology, 32, 78–91.
Hao, J., Song, F., Huang, F., Yang, C., Zhang, Z., Zheng, Y., et al. (2007). Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. Journal of Industrial Microbiology and Biotechnology, 34, 233–240.
D’Souza-Ticlo, D., Garg, S., & Raghukumar, C. (2009). Effects and interactions of medium components on laccase from a marine-derived fungus using response surface methodology. Marine Drugs, 7, 672–688.
Brar, S. K., Gassara, F., Tyagi, R. D., Verma, M., & Surampalli, R. Y. (2010). Screening of agro-industrial wastes to produce ligninolytic enzymes by Phanerochaete chrysosporium. Biochemical Engineering Journal, 49, 388–394.
Thygesen, A., Thomsen, A. B., Schmidt, A. S., Jørgensen, H., Ahring, B. K., & Olsson, L. (2003). Production of cellulose and hemicellulose-degrading enzymes by filamentous fungi cultivated on wet-oxidised wheat straw. Enzyme and Microbial Technology, 32, 606–615.
Dillon, A., & Camassola, M. (2007). Production of cellulases and hemicellulases by Penicillium echinulatum grown on pretreated sugar cane bagasse and wheat bran in solid-state fermentation. Journal of Applied Microbiology, 103, 2196–2204.
Singh, A., Tuteja, S., Singh, N., & Bishnoi, N. R. (2011). Enhanced saccharification of rice straw and hull by microwave-alkali pretreatment and lignocellulolytic enzyme production. Bioresource Technology, 102, 1773–1782.
Aboud, A. A. O., Kidunda, R. S., & Osarya, J. (2005). Potential of water hyacinth (Eicchornia crassipes) in ruminant nutrition in Tanzania. Livestock Research for Rural Development, 17.
Abdel-sabour, M. F. (2010). Water hyacinth: available and renewable resource. Electronic Journal of Environmental, Agricultural and Food Chemistry, 9, 1746–1759.
Ghosh, S., Kumar, A., & Singh, L. K. (2009). Bioconversion of lignocellulosic fraction of water-hyacinth (Eichhornia crassipes) hemicellulose acid hydrolysate to ethanol by Pichia stipitis. Bioresource Technology, 100, 3293–3297.
D’Annibale, A., Quaratino, D., Federici, F., Petruccioli, M., & Fenice, M. (2007). Production, purification and partial characterisation of a novel laccase from the white-rot fungus Panus tigrinus CBS 577.79. Anton Leeuwhoek International Journal of General and Molecular Microbiology, 91, 57–69.
Martin, C., Almazan, O., Marcet, M., & Jonsson, L. J. (2007). A study of three strategies for improving the fermentability of sugarcane bagasse hydrolysates for fuel ethanol production. International Sugar Journal, 109, 33.
Aguiar, M. M., Ferreira, L. F. R., & Monteiro, R. T. R. (2010). Use of vinasse and sugarcane bagasse for the production of enzymes by lignocellulolytic fungi. Brazilian Archives of Biology and Technology, 53, 1245–1254.
Pandey, A. (2003). Solid-state fermentation. Biochemical Engineering Journal, 13, 81–84.
Zheng, Y., Pan, Z., & Zhang, R. (2009). Overview of biomass pretreatment for cellulosic ethanol production. International Journal of Agricultural & Biological Engineering, 2, 51–68.
Torre, P., Aliakbarian, B., Rivas, B., Domínguez, J. M., & Converti, A. (2008). Release of ferulic acid from corn cobs by alkaline hydrolysis. Biochemical Engineering Journal, 40, 500–506.
Gianfreda, L., Xu, F., & Bollag, J.-M. (1999). Laccases: a useful group of oxidoreductive enzymes. Bioremediation Journal, 3, 1–26.
Maeda, R. N., Serpa, V. I., Rocha, V. A. L., Mesquita, R. A. A., Anna, L. M. M. S., de Castro, A. M., et al. (2011). Enzymatic hydrolysis of pretreated sugar cane bagasse using Penicillium funiculosum and Trichoderma harzianum cellulases. Process Biochemistry, 46, 1196–1201.
Chen, Y., Dong, B., Qin, W., & Xiao, D. (2010). Xylose and cellulose fractionation from corncob with three different strategies and separate fermentation of them to bioethanol. Bioresource Technology, 101, 6994–6999.
Zhang, Q., & Cai, W. (2008). Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass and Bioenergy, 32, 1130–1135.
Acknowledgements
This work was supported by National Natural Science Foundation of China, Scientific Technology Program of Zhejiang Province (2011C33016) and the Special Funds for Major State Basic Research Program of China (973 Program, 2007CB707805).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, HY., Xue, DS., Feng, XY. et al. Screening and Production of Ligninolytic Enzyme by a Marine-Derived Fungal Pestalotiopsis sp. J63. Appl Biochem Biotechnol 165, 1754–1769 (2011). https://doi.org/10.1007/s12010-011-9392-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-011-9392-y