Abstract
Background
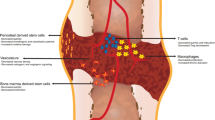
Poor fracture healing in geriatric populations is a significant source of morbidity, mortality, and cost to individuals and society; however, a fundamental biologic understanding of age-dependent healing remains elusive. The development of an aged-based fracture model system would allow for a mechanistic understanding that could guide future biologic treatments.
Questions/purposes
Using a small animal model of long-bone fracture healing based on chronologic age, we asked how aging affected (1) the amount, density, and proportion of bone formed during healing; (2) the amount of cartilage produced and the progression to bone during healing; (3) the callus structure and timing of the fracture healing; and (4) the behavior of progenitor cells relative to the observed deficiencies of geriatric fracture healing.
Methods
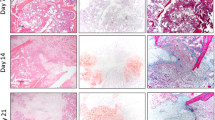
Transverse, traumatic tibial diaphyseal fractures were created in 5-month-old (n = 104; young adult) and 25-month-old (n = 107; which we defined as geriatric, and are approximately equivalent to 70–85 year-old humans) C57BL/6 mice. Fracture calluses were harvested at seven times from 0 to 40 days postfracture for micro-CT analysis (total volume, bone volume, bone volume fraction, connectivity density, structure model index, trabecular number, trabecular thickness, trabecular spacing, total mineral content, bone mineral content, tissue mineral density, bone mineral density, degree of anisotropy, and polar moment of inertia), histomorphometry (total callus area, cartilage area, percent of cartilage, hypertrophic cartilage area, percent of hypertrophic cartilage area, bone and osteoid area, percent of bone and osteoid area), and gene expression quantification (fold change).
Results
The geriatric mice produced a less robust healing response characterized by a pronounced decrease in callus amount (mean total volume at 20 days postfracture, 30.08 ± 11.53 mm3 versus 43.19 ± 18.39 mm3; p = 0.009), density (mean bone mineral density at 20 days postfracture, 171.14 ± 64.20 mg hydroxyapatite [HA]/cm3 versus 210.79 ± 37.60 mg HA/cm3; p = 0.016), and less total cartilage (mean cartilage area at 10 days postfracture, 101,279 ± 46,755 square pixels versus 302,167 ± 137,806 square pixels; p = 0.013) and bone content (mean bone volume at 20 days postfracture, 11.68 ± 3.18 mm3 versus 22.34 ± 10.59 mm3; p < 0.001) compared with the young adult mice. However, the amount of cartilage and bone relative to the total callus size was similar between the adult and geriatric mice (mean bone volume fraction at 25 days postfracture, 0.48 ± 0.10 versus 0.50 ± 0.13; p = 0.793), and the relative expression of chondrogenic (mean fold change in SOX9 at 10 days postfracture, 135 + 25 versus 90 ± 52; p = 0.221) and osteogenic genes (mean fold change in osterix at 20 days postfracture, 22.2 ± 5.3 versus 18.7 ± 5.2; p = 0.324) was similar. Analysis of mesenchymal cell proliferation in the geriatric mice relative to adult mice showed a decrease in proliferation (mean percent of undifferentiated mesenchymal cells staining proliferating cell nuclear antigen [PCNA] positive at 10 days postfracture, 25% ± 6.8% versus 42% ± 14.5%; p = 0.047).
Conclusions
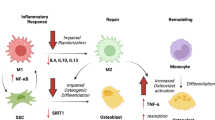
Our findings suggest that the molecular program of fracture healing is intact in geriatric mice, as it is in geriatric humans, but callus expansion is reduced in magnitude.
Clinical Relevance
Our study showed altered healing capacity in a relevant animal model of geriatric fracture healing. The understanding that callus expansion and bone volume are decreased with aging can help guide the development of targeted therapeutics for these difficult to heal fractures.








Similar content being viewed by others
References
Arias E. United States Life Tables, 2006. National Vital Statistics Reports. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_21.pdf. Accessed January 30, 2013.
Baldock PA, Need AG, Moore RJ, Durbridge TC, Morris HA. Discordance between bone turnover and bone loss: effects of aging and ovariectomy in the rat. J Bone Miner Res. 1999;14:1442–1448.
Bellows CG, Pei W, Jia Y, Heersche JN. Proliferation, differentiation and self-renewal of osteoprogenitors in vertebral cell populations from aged and young female rats. Mech Ageing Dev. 2003;124:747–757.
Bergman RJ, Gazit D, Kahn AJ, Gruber H, McDougall S, Hahn TJ. Age-related changes in osteogenic stem cells in mice. J Bone Miner Res. 1996;11:568–577.
Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2:97–101.
Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age-dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5845.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475.
Burkhardt R, Kettner G, Bohm W, Schmidmeier M, Schlag R, Frisch B, Mallmann B, Eisenmenger W, Gilg T. Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: a comparative histomorphometric study. Bone. 1987;8:157–164.
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561.
Christensen L, Iqbal S, Macarios D, Badamgarav E, Harley C. Cost of fractures commonly associated with osteoporosis in a managed-care population. J Med Econ. 2010;13:302–313.
Dishowitz MI, Mutyaba PL, Takacs JD, Barr AM, Engiles JB, Ahn J, Hankenson KD. Systemic inhibition of canonical notch signaling results in sustained callus inflammation and alters multiple phases of fracture healing. PloS One. 2013;8:e68726.
Dishowitz MI, Terkhorn SP, Bostic SA, Hankenson KD. Notch signaling components are upregulated during both endochondral and intramembranous bone regeneration. J Orthop Res. 2012;30:296–303.
Egermann M, Goldhahn J, Schneider E. Animal models for fracture treatment in osteoporosis. Osteoporos Int. 2005;16(suppl 2):S129–138.
Egermann M, Heil P, Tami A, Ito K, Janicki P, Von Rechenberg B, Hofstetter W, Richards PJ. Influence of defective bone marrow osteogenesis on fracture repair in an experimental model of senile osteoporosis. J Orthop Res. 2010;28:798–804.
Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003;33:387–398.
Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–1207.
Gruber R, Koch H, Doll BA, Tegtmeier F, Einhorn TA, Hollinger JO. Fracture healing in the elderly patient. Exp Gerontol. 2006;41:1080–1093.
Histing T, Kuntz S, Stenger D, Scheuer C, Garcia P, Holstein JH, Klein M, Pohlemann T, Menger MD. Delayed fracture healing in aged senescence-accelerated P6 mice. J Invest Surg. 2013;26:30–35.
Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell-derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis Rheum. 2009;60:813–823.
Lai JK, Lucas RM, Armstrong M, Banks E. Prospective observational study of physical functioning, physical activity, and time outdoors and the risk of hip fracture: A population-based cohort study of 158,057 older adults in the 45 and up study. J Bone Miner Res. 2013;28:2222–2231.
Lang TF, Sigurdsson S, Karlsdottir G, Oskarsdottir D, Sigmarsdottir A, Chengshi J, Kornak J, Harris TB, Sigurdsson G, Jonsson BY, Siggeirsdottir K, Eiriksdottir G, Gudnason V, Keyak JH. Age-related loss of proximal femoral strength in elderly men and women: the Age Gene/Environment Susceptibility Study—Reykjavik. Bone. 2012;50:743–748.
Leucht P, Jiang J, Cheng D, Liu B, Dhamdhere G, Fang MY, Monica SD, Urena JJ, Cole W, Smith LR, Castillo AB, Longaker MT, Helms JA. Wnt3a reestablishes osteogenic capacity to bone grafts from aged animals. J Bone Joint Surg Am. 2013;95:1278–1288.
Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307.
Meyer RA Jr, Meyer MH, Tenholder M, Wondracek S, Wasserman R, Garges P. Gene expression in older rats with delayed union of femoral fractures. J Bone Joint Surg Am. 2003;85:1243–1254.
Miedel E, Dishowitz MI, Myers MH, Dopkin D, Yu YY, Miclau TS, Marcucio R, Ahn J, Hankenson KD. Disruption of thrombospondin-2 accelerates ischemic fracture healing. J Orthop Res. 2013;31:935–943.
Mutyaba PL, Belkin NS, Lopas L, Gray CF, Dopkin D, Hankenson KD, Ahn J. Notch signaling in mesenchymal stem cells harvested from geriatric mice. J Orthop Trauma. 2014;28 (suppl 1):S20–23.
Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O’Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res. 2009;24:251–264.
National Institute on Aging. Aged Rodent Colonies Handbook. Strain Survival Information. U.S. Department of Health and Human Services. Available at: http://www.nia.nih.gov/research/dab/aged-rodent-colonies-handbook/strain-survival-information. Accessed January 30, 2013.
Parfitt AM, Han ZH, Palnitkar S, Rao DS, Shih MS, Nelson D. Effects of ethnicity and age or menopause on osteoblast function, bone mineralization, and osteoid accumulation in iliac bone. J Bone Miner Res. 1997;12:1864–1873.
Parfitt AM, Villanueva AR, Foldes J, Rao DS. Relations between histologic indices of bone formation: implications for the pathogenesis of spinal osteoporosis. J Bone Miner Res. 1995;10:466–473.
Silva MJ, Brodt MD, Ettner SL. Long bones from the senescence accelerated mouse SAMP6 have increased size but reduced whole-bone strength and resistance to fracture. J Bone Miner Res. 2002;17:1597–1603.
Stauber M, Muller R. Micro-computed tomography: a method for the non-destructive evaluation of the three-dimensional structure of biological specimens. Methods Mol Biol. 2008;455:273–292.
Taylor DK, Meganck JA, Terkhorn S, Rajani R, Naik A, O’Keefe RJ, Goldstein SA, Hankenson KD. Thrombospondin-2 influences the proportion of cartilage and bone during fracture healing. J Bone Miner Res. 2009;24:1043–1054.
van den Bergh JP, van Geel TA, Geusens PP. Osteoporosis, frailty and fracture: implications for case finding and therapy. Nat Rev Rheumatol. 2012;8:163–172.
Walsh WR, Sherman P, Howlett CR, Sonnabend DH, Ehrlich MG. Fracture healing in a rat osteopenia model. Clin Orthop Relat Res. 1997;342:218–227.
White BL, Fisher WD, Laurin CA. Rate of mortality for elderly patients after fracture of the hip in the 1980’s. J Bone Joint Surg Am. 1987;69:1335–1340.
Wronski TJ, Lowry PL, Walsh CC, Ignaszewski LA. Skeletal alterations in ovariectomized rats. Calcif Tissue Int. 1985;37:324–328.
Wronski TJ, Morey ER. Inhibition of cortical and trabecular bone formation in the long bones of immobilized monkeys. Clin Orthop Relat Res. 1983;181:269–276.
Xing Z, Lu C, Hu D, Miclau T 3rd, Marcucio RS. Rejuvenation of the inflammatory system stimulates fracture repair in aged mice. J Orthop Res. 2010;28:1000–1006.
Xing Z, Lu C, Hu D, Yu YY, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458.
Zhang YB, Zhong ZM, Hou G, Jiang H, Chen JT. Involvement of oxidative stress in age-related bone loss. J Surg Res. 2011;169:e37–42.
Acknowledgments
We thank Michael Karp MS and Brian Horwich MS (micro-CT data acquisition) Department of Bioengineering, University of Pennsylvania; Derek Dopkin BA (colony management) Department of Clinical Studies-New Bolton Center, University of Pennsylvania; Allison Williams BS (quantitative PCR) Department of Clinical Studies-New Bolton Center, University of Pennsylvania; and Michael Dishowitz PhD (experimental design and techniques) Department of Clinical Studies-New Bolton Center, University of Pennsylvania for their technical contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
One or more of the authors certify that they have received, during the study period, funding from NIH R03AG040670 (JA, KDH), American Geriatric Society Jahnigan Award (JA), McCabe Foundation Pilot Award (JA, KDH), and Penn Center for Musculoskeletal Disorders Pilot Award (NIH P30AR050950) (JA, KDH). One of the authors (KDH) certifies that he or she, or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of less than USD 10,000 from Skelegen (Philadelphia, PA, USA). One of the authors (JA) certifies that he or she, or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount less than USD 10,000 from Synthes Inc (West Chester, PA, USA), an amount of USD 10,000 to USD 100,000 from Merck & Co, Inc (Whitehouse Station, NJ, USA), and an amount less than USD 10,000 from U&I Corp (Uijeongbu-si, Gyeonggi-do, Korea).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at University of Pennsylvania, Philadelphia, PA, USA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Lopas, L.A., Belkin, N.S., Mutyaba, P.L. et al. Fractures in Geriatric Mice Show Decreased Callus Expansion and Bone Volume. Clin Orthop Relat Res 472, 3523–3532 (2014). https://doi.org/10.1007/s11999-014-3829-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-014-3829-x