Abstract
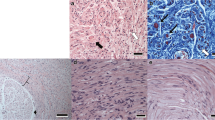
Bone morphogenic proteins (BMPs) may have neurotrophic functions but there is limited evidence of these functions in the peripheral nervous system. We therefore investigated the expression of BMPs and BMP receptors (BMPRs) in normal and injured peripheral nerves. In 10 of 15 Sprague-Dawley rats, a 3-mm segment of sciatic nerve was resected at the trifurcation in the thigh. One day (n = 5) and 7 days (n = 5) after transection, proximal and distal stumps were removed and immunohistochemically analyzed for BMP-2, -7, BMPR-1A, -1B, and -2. The other five animals served as normal controls. In normal nerves, BMP-2 expression was localized at Ranvier’s node, and BMP-7 and BMPR-1B were expressed in several axon-Schwann cell units, whereas other receptors were not expressed. After nerve transection, BMP-7 expression was upregulated at both proximal and distal stumps along with Schwann cell columns during Wallerian degeneration. BMPRs were also upregulated compared with the normal nerve. The upregulation in BMP expression after nerve transection suggests that BMPs may play a role in the healing response of the peripheral nerve.




Similar content being viewed by others
References
Althini S, Usoskin D, Kylberg A, ten Dijke P, Ebendal T. Bone morphogenetic protein signalling in NGF-stimulated PC12 cells. Biochem Biophys Res Commun. 2003;307:632–639.
Barde YA. Trophic factors and neuronal survival. Neuron. 1989;2:1525–1534.
Bengtsson H, Soderstrom S, Kylberg A, Charette MF, Ebendal T. Potentiating interactions between morphogenetic protein and neurotrophic factors in developing neurons. J Neurosci Res. 1998;53:559–568.
Chang CF, Lin SZ, Chiang YH, Morales M, Chou J, Lein P, Chen HL, Hoffer BJ, Wang Y. Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke. 2003;34:558–564.
Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663.
Ebendal T, Bengtsson H, Soderstrom S. Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res. 1998;51:139–146.
Helm GA, Alden TD, Sheehan JP, Kallmes D. Bone morphogenetic proteins and bone morphogenetic protein gene therapy in neurological surgery: a review. Neurosurgery. 2000;46:1213–1222.
Heumann R, Korsching S, Bandtlow C, Thoenen H. Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection. J Cell Biol. 1987;104:1623–1631.
Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594.
Ishibe T, Nakayama T, Aoyama T, Nakamura T, Toguchida. Neuronal differentiation of synovial sarcoma and its therapeutic application. Clin Orthop Relat Res. 2008;466:2147–2155.
Kalbermatten DF, Erba P, Mahay D, Wiberg M, Pierer G, Terenghi G. Schwann cell strip for peripheral nerve repair. J Hand Surg Eur Vol. 2008;33:587–594.
Kinameri E, Matsuoka I. Autocrine action of BMP2 regulates expression of GDNF-mRNA in sciatic Schwann cells. Brain Res Mol Brain Res. 2003;117:221–227.
Lara-Ramirez R, Segura-Anaya E, Martinez-Gomez A, Dent MAR. Expression of interleukin-6 receptor α in normal and injured rat sciatic nerve. Neuroscience. 2008;152:601–608.
Lin SZ, Hoffer BJ, Kaplan P, Wang Y. Osteogenic protein-1 protects against cerebral infarction induced by MCA ligation in adult rats. Stroke. 1999;30:126–133.
Lonn P, Zaia K, Israelsson C, Althini S, Usoskin D, Kylberg A, Ebendal T. BMP enhances transcriptional responses to NGF during PC12 cell differentiation. Neurochem Res. 2005;30:753–765.
Lundborg G, Rosén B. Hand function after nerve repair. Acta Physiol (Oxf). 2007;189:207–217.
Maki Y, Yoshizu T, Tsubokawa N. Selective regeneration of motor and sensory axons in an experimental peripheral nerve model without endorgans. Scand J Plast Reconstr Surg Hand Surg. 2005;39:257–260.
Meyer M, Matsuoka I, Wetmore C, Olson L, Thoenen H. Enhanced synthesis of brain-derived neurotrophic factor in the lesioned peripheral nerve: different mechanisms are responsible for the regulation of BDNF and NGF mRNA. J Cell Biol. 1992;119:45–54.
Okuyama N, Kiryu-Seo S, Kiyama H. Altered expression of Smad family members in injured motor neurons of rat. Brain Res. 2007;1132:36–41.
Perides G, Jensen FE, Edgecomb P, Rueger DC, Charness ME. Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci Lett. 1995;187:21–24.
Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development. 1996;122:2079–2088.
Saito H, Dahlin LB. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008;9:88.
Schafer DP, Rasband MN. Glial regulation of the axonal membrane at nodes of Ranvier. Curr Opin Neurobiol. 2006;16:508–514.
Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787.
Thoenen H. The changing scene of neurotrophic factors. Trends Neurosci. 1991;14:165–170.
Trupp M, Rydén M, Jörnvall H, Funakoshi H, Timmusk T, Arenas E, Ibáñez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148.
Turgut M, Oktem G, Uysal A, Yurtseven ME. Immunohistochemical profile of transforming growth factor-beta1 and basic fibroblast growth factor in sciatic nerve anastomosis following pinealectomy and exogenous melatonin administration in rats. J Clin Neurosci. 2006;13:753–758.
Varley JE, Maxwell GD. BMP-2 and BMP-4, but not BMP-6, increase the number of adrenergic cells which develop in quail trunk neural crest cultures. Exp Neurol. 1996;140:84–94.
Varley JE, Wehby RG, Rueger DC, Maxwell GD. Number of adrenergic and islet-1 immunoreactive cells is increased in avian trunk neural crest cultures in the presence of human recombinant osteogenic protein-1. Dev Dyn. 1995;203:434–447.
Wang YL, Wang DZ, Nie X, Lei DL, Liu YP, Zhang YJ, Suwa F, Tamada Y, Fang YR, Jin Y. The role of bone morphogenetic protein-2 in vivo in regeneration of peripheral nerves. Br J Oral Maxillofac Surg. 2007;45:197–202.
Yamada M, Akeda K, Asanuma K, Thonar EJ, An HS, Uchida A, Masuda K. Effect of osteogenic protein-1 on the matrix metabolism of bovine tendon cells. J Orthop Res. 2008;26:42–48.
Zhang D, Mehler MF, Song Q, Kessler JA. Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci. 1998;18:3314–3326.
Author information
Authors and Affiliations
Corresponding author
Additional information
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
About this article
Cite this article
Tsujii, M., Akeda, K., Iino, T. et al. Are BMPs Involved in Normal Nerve and Following Transection?: A Pilot Study. Clin Orthop Relat Res 467, 3183–3189 (2009). https://doi.org/10.1007/s11999-009-1009-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11999-009-1009-1