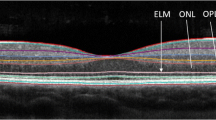
Opinion statement
Retinae of patients with multiple sclerosis (MS), as part of the central nervous system (CNS), display inflammatory and neurodegenerative changes. There is increasing evidence suggesting that retinal changes, and in particular neurodegeneration, mirror global CNS alterations in MS. Spectral domain optical coherence tomography (SD-OCT) is an inexpensive, rapid, non-invasive, and reproducible imaging technique that generates high-resolution images of tissues such as the retina. An advantage of SD-OCT over magnetic resonance imaging techniques in the assessment of neurodegeneration may be its sensitivity to capture changes at the individual patient level. Several studies demonstrate that changes within the inner retina (primarily as a reflection of optic neuropathy), as assessed by OCT, correlate with reduced quality of life, visual dysfunction, and global disability in MS. Moreover, longitudinal studies suggest that inner retinal thinning is an early phenomenon in MS and that retinal thinning may occur independent of previous symptomatic episodes of optic neuritis, significantly correlating with inflammatory disease. Preliminary studies suggest that MS disease-modifying therapies may have differential effects on OCT-determined rates of retinal atrophy, supporting a potential utility for OCT to investigate the neuroprotective benefits of disease-modifying therapies in MS, as well as an outcome in trials of putatively neuroprotective strategies.


Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–81.
Hrynchak P, Simpson T. Optical coherence tomography: an introduction to the technique and its use. Optom Vis Sci. 2000;77:347–56.
Frohman EM, Fujimoto JG, Frohman TC, Calabresi PA, Cutter G, Balcer LJ. Optical coherence tomography: a window into the mechanisms of multiple sclerosis. Nat Clin Pract Neurol. 2008;4:664–75.
Warner CV, Syc SB, Stankiewicz AM, Hiremath G, Farrell SK, Crainiceanu CM, et al. The impact of utilizing different optical coherence tomography devices for clinical purposes and in multiple sclerosis trials. PLoS One. 2011;6:e22947.
• Saidha S, Syc SB, Durbin MK, Eckstein C, Oakley JD, Meyer SA, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17:1449–63. This was one of the first studies to demonstrate that GCIP thickness may have superior structure–function relationships with disability and visual function in MS as compared to RNFL thickness.
Saidha S, Syc SB, Ibrahim MA, Eckstein C, Warner CV, Farrell SK, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134:518–33.
• Syc SB, Saidha S, Newsome SD, Ratchford JN, Levy M, Ford E, et al. Optical coherence tomography segmentation reveals ganglion cell layer pathology after optic neuritis. Brain. 2012;135:521–33. This paper showed how the ganglion cell layer reflects neurodegeneration in acute optic neuritis without confounding swelling and introduced this as a novel parameter to track optic neuritis severity.
Seigo MA, Sotirchos ES, Newsome S, Babiarz A, Eckstein C, Ford E, et al. In vivo assessment of retinal neuronal layers in multiple sclerosis with manual and automated optical coherence tomography segmentation techniques. J Neurol. 2012;259:2119–30.
Lang A, Carass A, Hauser M, Sotirchos ES, Calabresi PA, Ying HS, et al. Retinal layer segmentation of macular OCT images using boundary classification. Biomed Opt Express. 2013;4:1133–52.
Lang A, Carass A, Sotirchos E, Calabresi P, Prince JL. Segmentation of retinal OCT images using a random forest classifier. Proc SPIE Int Soc Opt Eng 2013;8669.
• Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain. 2010;133:1591–601. This oculo-histopathology study of MS eyes shows that 79% of MS eyes have a dropout of ganglion cell neurons. Moreover, 40% of MS eyes have a dropout of neurons within the inner nuclear layer. In addition, activated microglia can be seen in the retinas of MS patients.
Ratchford JN, Saidha S, Sotirchos ES, Oh JA, Seigo MA, Eckstein C, et al. Active MS is associated with accelerated retinal ganglion cell/inner plexiform layer thinning. Neurology. 2013;80:47–54.
• Saidha S, Sotirchos ES, Ibrahim MA, Crainiceanu CM, Gelfand JM, Sepah YJ, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–72. This study provides evidence that changes in the inner nuclear layer thickness/volume are related with inflammatory activity in patients with multiple sclerosis.
Knier B, Schmidt P, Aly L, Buck D, Berthele A, Muhlau M, Zimmer C, Hemmer B, Korn T. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016 Aug 30. pii: aww219. [Epub ahead of print]
• Gabilondo I, Martinez-Lapiscina EH, Fraga-Pumar E, Ortiz-Perez S, Torres-Torres R, Andorra M, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77:517–28. This paper provides an extensive investigation in retinal changes after optic neuritis.
Al-Louzi OA, Bhargava P, Newsome SD, Balcer LJ, Frohman EM, Crainiceanu C, et al. Outer retinal changes following acute optic neuritis. Mult Scler. 2016;22:362–72.
Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, et al. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci. 1999;40:2520–7.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Barkhof F, Calabresi PA, Miller DH, Reingold SC. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–66.
Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, Garcia-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology. 2007;68:1488–94.
Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69:1603–9.
Dorr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF, et al. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One. 2011;6:e18132.
• Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, et al. Optical coherence tomography reflects brain atrophy in multiple sclerosis: a four-year study. Ann Neurol. 2015;78:801–13. This study provides the strongest evidence to support the association between retinal and brain atrophy in patients with multiple sclerosis.
• Costello F, Coupland S, Hodge W, Lorello GR, Koroluk J, Pan YI, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59:963–9. This study provides the first evidence of a nonlinear association between retinal thickness and clinical outcome: a threshold of RNFL thickness (75 μm), below which RNFL measurements predicted persistent visual dysfunction after acute optic neuritis.
Naismith RT, Xu J, Tutlam NT, Snyder A, Benzinger T, Shimony J, et al. Disability in optic neuritis correlates with diffusion tensor-derived directional diffusivities. Neurology. 2009;72:589–94.
Kolbe SC, Marriott M, Walt A, Fielding J, Klistorner A, Mitchell PJ, et al. Diffusion tensor imaging correlates of visual impairment in multiple sclerosis and chronic optic neuritis. Invest Ophthalmol Vis Sci. 2012;53:825–32.
Wang Y, van der Walt A, Paine M, Klistorner A, Butzkueven H, Egan GF, et al. Optic nerve magnetisation transfer ratio after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2012;7:e52291.
• Gabilondo I, Martinez-Lapiscina EH, Martinez-Heras E, Fraga-Pumar E, Llufriu S, Ortiz S, et al. Trans-synaptic axonal degeneration in the visual pathway in multiple sclerosis. Ann Neurol. 2014;75:98–107. This study provided evidence to support the potential role of trans-synaptic mechanisms of neurodegeneration in patients with multiple sclerosis.
• Pfueller CF, Brandt AU, Schubert F, Bock M, Walaszek B, Waiczies H, et al. Metabolic changes in the visual cortex are linked to retinal nerve fiber layer thinning in multiple sclerosis. PLoS One. 2011;6:e18019. This study provided evidence to support the potential role of trans-synaptic mechanisms of neurodegeneration in patients with multiple sclerosis.
Reich DS, Smith SA, Gordon-Lipkin EM, Ozturk A, Caffo BS, Balcer LJ, et al. Damage to the optic radiation in multiple sclerosis is associated with retinal injury and visual disability. Arch Neurol. 2009;66:998–1006.
Klistorner A, Sriram P, Vootakuru N, Wang C, Barnett MH, Garrick R, et al. Axonal loss of retinal neurons in multiple sclerosis associated with optic radiation lesions. Neurology. 2014;82:2165–72.
Sinnecker T, Oberwahrenbrock T, Metz I, Zimmermann H, Pfueller CF, Harms L, et al. Optic radiation damage in multiple sclerosis is associated with visual dysfunction and retinal thinning—an ultrahigh-field MR pilot study. Eur Radiol. 2015;25:122–31.
Scheel M, Finke C, Oberwahrenbrock T, Freing A, Pech LM, Schlichting J, et al. Retinal nerve fibre layer thickness correlates with brain white matter damage in multiple sclerosis: a combined optical coherence tomography and diffusion tensor imaging study. Mult Scler. 2014;20:1904–7.
Saidha S, Sotirchos ES, Oh J, Syc SB, Seigo MA, Shiee N, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70:34–43.
Young KL, Brandt AU, Petzold A, Reitz LY, Lintze F, Paul F, et al. Loss of retinal nerve fibre layer axons indicates white but not grey matter damage in early multiple sclerosis. Eur J Neurol. 2013;20:803–11.
Oh J, Sotirchos ES, Saidha S, Whetstone A, Chen M, Newsome SD, et al. Relationships between quantitative spinal cord MRI and retinal layers in multiple sclerosis. Neurology. 2015;84:720–8.
• Zimmermann H, Freing A, Kaufhold F, Gaede G, Bohn E, Bock M, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler. 2013;19:443–50. This study demonstrates that the global relationships reflected by retinal measures in MS are skewed/masked by prior optic neuritis possibly due to disproportionate localized retinal tissue injury following optic neuritis.
Knier B, Berthele A, Buck D, Schmidt P, Zimmer C, Muhlau M, et al. Optical coherence tomography indicates disease activity prior to clinical onset of central nervous system demyelination. Mult Scler. 2016;22:893–900.
Costello F, Hodge W, Pan YI, Freedman M, DeMeulemeester C. Differences in retinal nerve fiber layer atrophy between multiple sclerosis subtypes. J Neurol Sci. 2009;281:74–9.
Costello F, Hodge W, Pan YI, Eggenberger E, Freedman MS. Using retinal architecture to help characterize multiple sclerosis patients. Can J Ophthalmol. 2010;45:520–6.
Gelfand JM, Goodin DS, Boscardin WJ, Nolan R, Cuneo A, Green AJ. Retinal axonal loss begins early in the course of multiple sclerosis and is similar between progressive phenotypes. PLoS One. 2012;7:e36847.
• Oberwahrenbrock T, Schippling S, Ringelstein M, Kaufhold F, Zimmermann H, Keser N, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. This large cross-sectional study summarizes time domain OCT findings in different multiple sclerosis subtypes.
Oberwahrenbrock T, Ringelstein M, Jentschke S, Deuschle K, Klumbies K, Bellmann-Strobl J, et al. Retinal ganglion cell and inner plexiform layer thinning in clinically isolated syndrome. Mult Scler. 2013;19:1887–95.
Lange AP, Zhu F, Sayao AL, Sadjadi R, Alkabie S, Traboulsee AL, et al. Retinal nerve fiber layer thickness in benign multiple sclerosis. Mult Scler. 2013;19:1275–81.
Pulicken M, Gordon-Lipkin E, Balcer LJ, Frohman E, Cutter G, Calabresi PA. Optical coherence tomography and disease subtype in multiple sclerosis. Neurology. 2007;69:2085–92.
Burggraaff MC, Trieu J, de Vries-Knoppert WA, Balk L, Petzold A. The clinical spectrum of microcystic macular edema. Invest Ophthalmol Vis Sci. 2014;55:952–61.
• Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135:1786–93. This was the first study to demonstrate macular microcystoid changes in MS and identify an association between the presence of such retinal findings and increased disability in MS.
McDonald WI, Barnes D. The ocular manifestations of multiple sclerosis. 1. Abnormalities of the afferent visual system. J Neurol Neurosurg Psychiatry. 1992;55:747–52.
Balcer LJ, Baier ML, Cohen JA, Kooijmans MF, Sandrock AW, Nano-Schiavi ML, et al. Contrast letter acuity as a visual component for the Multiple Sclerosis Functional Composite. Neurology. 2003;61:1367–73.
Newman N. Multiple sclerosis and related demyelinating diseases. In: Miller NR, Newman NJ, editors. Walsh and Hoyt’s clinical neuro-ophthalmology. 5th ed. Baltimore: Williams & Wilkins; 1998;5539-5676
Rudick RA, Miller D, Clough JD, Gragg LA, Farmer RG. Quality of life in multiple sclerosis. Comparison with inflammatory bowel disease and rheumatoid arthritis. Arch Neurol. 1992;49:1237–42.
Ma SL, Shea JA, Galetta SL, Jacobs DA, Markowitz CE, Maguire MG, et al. Self-reported visual dysfunction in multiple sclerosis: new data from the VFQ-25 and development of an MS-specific vision questionnaire. Am J Ophthalmol. 2002;133:686–92.
Walter SD, Ishikawa H, Galetta KM, Sakai RE, Feller DJ, Henderson SB, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119:1250–7.
Lampert EJ, Andorra M, Torres-Torres R, Ortiz-Perez S, Llufriu S, Sepulveda M, et al. Color vision impairment in multiple sclerosis points to retinal ganglion cell damage. J Neurol. 2015;262:2491–7.
• Baier ML, Cutter GR, Rudick RA, Miller D, Cohen JA, Weinstock-Guttman B, et al. Low-contrast letter acuity testing captures visual dysfunction in patients with multiple sclerosis. Neurology. 2005;64:992–5. This article described the important role of low-contrast letter acuity test to capture visual dysfunction in patients with multiple sclerosis.
Sabadia SB, Nolan RC, Galetta KM, Narayana KM, Wilson JA, Calabresi PA, et al. 20/40 or better visual acuity after optic neuritis: not as good as we once thought? J Neuroophthalmol. 2016;36:369–76.
Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, et al. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006;113:324–32.
• Talman LS, Bisker ER, Sackel DJ, Long Jr DA, Galetta KM, Ratchford JN, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67:749–60. In this study, the authors found that MS patients with increasing reduction in retinal nerve fiber layer thickness had increasing loss of visual function over time.
• Martinez-Lapiscina EH, Ortiz-Perez S, Fraga-Pumar E, Martinez-Heras E, Gabilondo I, Llufriu S, et al. Colour vision impairment is associated with disease severity in multiple sclerosis. Mult Scler. 2014;20:1207–16. This study demonstrates that MS patients developing impairment of color vision over time have more severe MS.
Ortiz-Perez S, Andorra M, Sanchez-Dalmau B, Torres-Torres R, Calbet D, Lampert EJ, et al. Visual field impairment captures disease burden in multiple sclerosis. J Neurol. 2016;263:695–702.
Cheng H, Laron M, Schiffman JS, Tang RA, Frishman LJ. The relationship between visual field and retinal nerve fiber layer measurements in patients with multiple sclerosis. Invest Ophthalmol Vis Sci. 2007;48:5798–805.
Cole SR, Beck RW, Moke PS, Gal RL, Long DT. The National Eye Institute Visual Function Questionnaire: experience of the ONTT. Optic Neuritis Treatment Trial. Investig Ophthalmol Vis Sci. 2000;41:1017–21.
Noble J, Forooghian F, Sproule M, Westall C, O’Connor P. Utility of the National Eye Institute VFQ-25 questionnaire in a heterogeneous group of multiple sclerosis patients. Am J Ophthalmol. 2006;142:464–8.
Raphael BA, Galetta KM, Jacobs DA, Markowitz CE, Liu GT, Nano-Schiavi ML, et al. Validation and test characteristics of a 10-item neuro-ophthalmic supplement to the NEI-VFQ-25. Am J Ophthalmol. 2006;142:1026–35.
Mowry EM, Loguidice MJ, Daniels AB, Jacobs DA, Markowitz CE, Galetta SL, et al. Vision related quality of life in multiple sclerosis: correlation with new measures of low and high contrast letter acuity. J Neurol Neurosurg Psychiatry. 2009;80:767–72.
Toledo J, Sepulcre J, Salinas-Alaman A, Garcia-Layana A, Murie-Fernandez M, Bejarano B, et al. Retinal nerve fiber layer atrophy is associated with physical and cognitive disability in multiple sclerosis. Mult Scler. 2008;14:906–12.
Siepman TA, Bettink-Remeijer MW, Hintzen RQ. Retinal nerve fiber layer thickness in subgroups of multiple sclerosis, measured by optical coherence tomography and scanning laser polarimetry. J Neurol. 2010;257:1654–60.
Abalo-Lojo JM, Limeres CC, Gomez MA, Baleato-Gonzalez S, Cadarso-Suarez C, Capeans-Tome C, et al. Retinal nerve fiber layer thickness, brain atrophy, and disability in multiple sclerosis patients. J Neuroophthalmol. 2014;34:23–8.
Behbehani R, Al-Hassan AA, Al-Khars A, Sriraman D, Alroughani R. Retinal nerve fiber layer thickness and neurologic disability in relapsing-remitting multiple sclerosis. J Neurol Sci. 2015;359:305–8.
• Martinez-Lapiscina EH, Arnow S, Wilson JA, Saidha S, Preiningerova JL, Oberwahrenbrock T, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15:574–84. This study provides the strongest evidence so far to support the role of OCT as a predictor of disability worsening in patients with multiple sclerosis.
El Ayoubi NK, Ghassan S, Said M, Allam J, Darwish H, Khoury SJ. Retinal measures correlate with cognitive and physical disability in early multiple sclerosis. J Neurol. 2016;263:2287–95.
Anhoque CF, Biccas-Neto L, Domingues SC, Teixeira AL, Domingues RB. Cognitive impairment and optic nerve axonal loss in patients with clinically isolated syndrome. Clin Neurol Neurosurg. 2013;115:1032–5.
Wieder L, Gade G, Pech LM, Zimmermann H, Wernecke KD, Dorr JM, et al. Low contrast visual acuity testing is associated with cognitive performance in multiple sclerosis: a cross-sectional pilot study. BMC Neurol. 2013;13:167.
Garcia-Martin E, Rodriguez-Mena D, Herrero R, Almarcegui C, Dolz I, Martin J, et al. Neuro-ophthalmologic evaluation, quality of life, and functional disability in patients with MS. Neurology. 2013;81:76–83.
Edgar JM, Griffiths IR. White matter structure—chapter 5: a microscopist’s view. Elsevier 2009; 74-103.
Serbecic N, Aboul-Enein F, Beutelspacher SC, Vass C, Kristoferitsch W, Lassmann H, et al. High resolution spectral domain optical coherence tomography (SD-OCT) in multiple sclerosis: the first follow up study over two years. PLoS One. 2011;6:e19843.
Narayanan D, Cheng H, Bonem KN, Saenz R, Tang RA, Frishman LJ. Tracking changes over time in retinal nerve fiber layer and ganglion cell-inner plexiform layer thickness in multiple sclerosis. Mult Scler. 2014;20:1331–41.
Graham EC, You Y, Yiannikas C, Garrick R, Parratt J, Barnett MH, et al. Progressive loss of retinal ganglion cells and axons in nonoptic neuritis eyes in multiple sclerosis: a longitudinal optical coherence tomography study. Invest Ophthalmol Vis Sci. 2016;57:2311–7.
Balk LJ, Cruz-Herranz A, Albrecht P, Arnow S, Gelfand JM, Tewarie P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263:1323–31.
Nguyen A-L, Lam J, White R, Carruthers R, Traboulsee A. Prospective study of retinal nerve fibre layer thickness in alemtuzumab treated multiple sclerosis patients. Neurology 2016.
• Button J, Al-Louzi O, Lang A, Bhargava P, Newsome SD, Frohman T, et al. Disease-modifying therapies modulate retinal atrophy in multiple sclerosis: a retrospective study. Neurology. 2017;88(6):525–32. This study highlights the potential role of OCT in monitoring response to MS therapies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Alexander U. Brandt received funding from the German Federal Ministry for Economic Affairs and Energy (BMWi Exist 03EFEBE079).
Elena H. Martinez-Lapiscina received funding from the Instituto de Salud Carlos III, Spain, and Fondo Europeo de Desarrollo Regional (FEDER; JR16/00006), Grant for MS Innovation and Marató TV3 Charitable Foundation.
Rachel Nolan declares no funding.
Shiv Saidha received funding from the Race to Erase MS and Genentech Corporation.
Conflict of Interest
Alexander U. Brandt has received travel reimbursement from Bayer, Biogen, Teva, and Novartis and consulting or speaker honoraria from Biogen, Teva, Heidelberg Engineering, Motognosis, and Nexus. He is a member of the working committee of International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Elena H. Martinez-Lapiscina is a researcher in the OCTIMS Study, an observational study (which involves no specific drugs) to validate SD-OCT as a biomarker for multiple sclerosis, sponsored by Novartis. She has received speaking honoraria from Biogen and Genzyme and travel reimbursement from Genzyme, Roche, for international and national meetings over the last 3 years. She is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Rachel Nolan is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Shiv Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology, consulting fees from Axon Advisors LLC, speaking honoraria from the National Association of Managed Care Physicians, Family Medicine Foundation of West Virginia, and Advanced Studies in Medicine, and served on scientific advisory boards for Biogen-Idec, Genzyme, Genentech Corporation, and Novartis. He received research support from the Race to Erase MS and Genentech Corporation. He is a member of the working committee of the International Multiple Sclerosis Visual System (IMSVISUAL) Consortium.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Multiple Sclerosis and Related Disorders
Alexander U. Brandt, Elena H. Martinez-Lapiscina, Rachel Nolan and Shiv Saidha contributed equally to this work.
Rights and permissions
About this article
Cite this article
Brandt, A.U., Martinez-Lapiscina, E.H., Nolan, R. et al. Monitoring the Course of MS With Optical Coherence Tomography. Curr Treat Options Neurol 19, 15 (2017). https://doi.org/10.1007/s11940-017-0452-7
Published:
DOI: https://doi.org/10.1007/s11940-017-0452-7