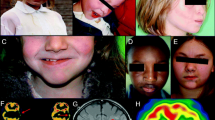
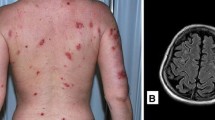
Abstract
Purpose of Review
To describe diverse neurologic and neuroradiologic presentations of two rare, immunologically mediated skin conditions: Sweet disease and localized scleroderma (morphea).
Recent Findings
Core syndromes of neuro-Sweet disease (NSD) are steroid responsiveness, recurrent meningitis, and encephalitis. Focal neurologic, neuro-vascular, and neuro-ophthalmologic syndromes have been reported recently in NSD. A variety of steroid-sparing treatments and biologics have been used for relapsing NSD. Localized craniofacial scleroderma is associated with seizures, headaches, and less commonly, focal deficits and cognitive decline. Immunosuppressive therapy may be required in patients with disease progression; some refractory cases have responded to IL-6 inhibition.
Summary
Our review provides an up-to-date reference for neurologists faced with a patient with a history or skin findings consistent with Sweet disease or localized scleroderma. We hope that it will stimulate collaborative studies aimed at unraveling the pathogenesis of these disorders, better characterization of their neurologic manifestations, and discovery of optimal therapeutic solutions.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hurko O, Provost TT. Neurology and the skin. J Neurol Neurosurg Psychiatry. 1999;66(4):417–30.
Nelson CA, Stephen S, Ashchyan HJ, James WD, Micheletti RG, Rosenbach M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behcet disease. J Am Acad Dermatol. 2018;79(6):987–1006. https://doi.org/10.1016/j.jaad.2017.11.064.
Galaria NA, Junkins-Hopkins JM, Kligman D, James WD. Neutrophilic dermatosis of the dorsal hands: pustular vasculitis revisited. J Am Acad Dermatol. 2000;43(5 Pt 1):870–4. https://doi.org/10.1067/mjd.2000.109286.
•• Drago F, Ciccarese G, Agnoletti AF, Sarocchi F, Parodi A. Neuro sweet syndrome: a systematic review. A rare complication of Sweet syndrome. Acta Neurol Belg. 2017;117(1):33–42. https://doi.org/10.1007/s13760-016-0695-1. Excellent systematic review of Neuro Sweet syndrome, including imaging, pathology and treatment approach.
Kandula S, Burke WS, Goldfarb JN. Clindamycin-induced Sweet syndrome. J Am Acad Dermatol. 2010;62(5):898–900. https://doi.org/10.1016/j.jaad.2009.03.050.
Thompson DF, Montarella KE. Drug-induced Sweet’s syndrome. Ann Pharmacother. 2007;41(5):802–11. https://doi.org/10.1345/aph.1H563.
Clark BM, Homeyer DC, Glass KR, D'Avignon LC. Clindamycin-induced Sweet’s syndrome. Pharmacotherapy. 2007;27(9):1343–6. https://doi.org/10.1592/phco.27.9.1343.
Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10(5):301–12. https://doi.org/10.2165/11310730-000000000-00000.
Hisanaga K, Hosokawa M, Sato N, Mochizuki H, Itoyama Y, Iwasaki Y. “Neuro-Sweet disease”: benign recurrent encephalitis with neutrophilic dermatosis. Arch Neurol. 1999;56(8):1010–3.
Hisanaga K, Iwasaki Y, Itoyama Y. Neuro-Sweet Disease Study G. Neuro-Sweet disease: clinical manifestations and criteria for diagnosis. Neurology. 2005;64(10):1756–61. https://doi.org/10.1212/01.WNL.0000161848.34159.B5.
Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of neuro-Behcet’s disease: international consensus recommendations. J Neurol. 2014;261(9):1662–76. https://doi.org/10.1007/s00415-013-7209-3.
Akman-Demir G, Serdaroglu P, Tasci B. Clinical patterns of neurological involvement in Behcet’s disease: evaluation of 200 patients. The Neuro-Behcet Study Group. Brain. 1999;122(Pt 11):2171–82.
Kato T, Kunikata N, Taira H, Kobayashi N, Tanji K, Endo M. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) with nodular episcleritis and polyneuropathy. Int J Dermatol. 2002;41(2):107–9.
Cala CM, Kole L, Sami N. Bilateral sensorineural hearing loss and polyneuropathy in a patient with Sweet’s syndrome. Case Rep Otolaryngol. 2015;2015:751538–3. https://doi.org/10.1155/2015/751538.
Taravati P. Neuro-sweet disease causing orbital inflammation. Neuroophthalmology. 2015;39(1):42–5. https://doi.org/10.3109/01658107.2014.961090.
Lobo AM, Stacy R, Cestari D, Stone JH, Jakobiec FA, Sobrin L. Optic nerve involvement with panuveitis in Sweet syndrome. Ocul Immunol Inflamm. 2011;19(3):167–70. https://doi.org/10.3109/09273948.2011.560411.
Sobol UA, Sherman KL, Smith J, Nagda SN, Micetich K, Nickoloff BJ, et al. Sweet’s syndrome with neurologic manifestations in a patient with esophageal adenocarcinoma: case report and review of the literature. Int J Dermatol. 2009;48(10):1062–5.
Ohori N, Kinoshita T, Toda K, Ohta S, Yoshimura T. A case of Sweet’s syndrome (acute febrile neutrophilic dermatosis) showing transient jargon aphasia. Rinsho Shinkeigaku. 1999;39(11):1156–9.
Macorig G, Pessa ME, Barbarino G, Scalise S, D'Agostini S, Valente M, et al. Neuro-Sweet disease: a diagnostic challenge. J Neurol Sci. 2016;371:42–4. https://doi.org/10.1016/j.jns.2016.10.012.
Makimoto G, Manabe Y, Yamakawa C, Fujii D, Ikeda-Sakai Y, Narai H, et al. Two cases of possible neuro-Sweet disease with meningoencephalitis as the initial manifestation. Neurol Int. 2012;4(1):e5. https://doi.org/10.4081/ni.2012.e5.
Niwa F, Tokuda T, Kimura M, Azuma Y, Mizuno T, Nakagawa M. Self-remitting and reversible parkinsonism associated with neuro-Sweet disease. Intern Med. 2010;49(12):1201–4.
Fukushima K, Hineno A, Kodaira M, Machida K, Ishii W, Kaneko T, et al. Reversible extensive leukoencephalopathy in Sweet disease: a case report. J Neurol Sci. 2008;275(1–2):178–80. https://doi.org/10.1016/j.jns.2008.08.006.
Kimura A, Sakurai T, Koumura A, Suzuki Y, Tanaka Y, Hozumi I, et al. Longitudinal analysis of cytokines and chemokines in the cerebrospinal fluid of a patient with neuro-Sweet disease presenting with recurrent encephalomeningitis. Intern Med. 2008;47(3):135–41.
Marien P, Tops W, Crols R, Jonkers R, De Deyn PP, Verhoeven J. Grammar disruption in a patient with neuro-Sweet syndrome. Neurocase. 2012;18(3):235–47. https://doi.org/10.1080/13554794.2011.588178.
Das AS, Conway SE, Unizony SH, Bouffard MA, Rost NS, Venna N. Pearls & oysters: neuro-Sweet disease presenting as ischemic stroke and aseptic meningitis. Neurology. 2018;91(23):e2197–e9. https://doi.org/10.1212/WNL.0000000000006628.
Sakamoto M, Kurimoto T, Mori S, Ueda K, Keshi Y, Yamada Y, et al. Vasculitis with superior ophthalmic vein thrombosis compatible with neuro-neutrophilic disease. Am J Ophthalmol Case Rep. 2018;12:39–44. https://doi.org/10.1016/j.ajoc.2018.08.005.
Sato K, Tsunoda K, Yamashita T, Takemoto M, Hishikawa N, Ohta Y, et al. A case of very long longitudinally extensive transverse myelitis (LETM) with necrotizing Vasculitis. J Neurol Sci. 2017;373:152–4. https://doi.org/10.1016/j.jns.2016.12.040.
Sudhakar P, Tobin S, Connor WO, Kedar S. Neuro-ophthalmic presentation of neuro-Sweet disease. Neuroophthalmology. 2017;41(4):202–6. https://doi.org/10.1080/01658107.2017.1291687.
Maxwell G, Archibald N, Turnbull D. Neuro-Sweet’s disease. Pract Neurol. 2012;12(2):126–30. https://doi.org/10.1136/practneurol-2011-000067.
Magro CM, De Moraes E, Burns F. Sweet’s syndrome in the setting of CD34-positive acute myelogenous leukemia treated with granulocyte colony stimulating factor: evidence for a clonal neutrophilic dermatosis. J Cutan Pathol. 2001;28(2):90–6.
• Charlson R, Kister I, Kaminetzky D, Shvartsbeyn M, Meehan SA, Mikolaenko I. CNS neutrophilic vasculitis in neuro-Sweet disease. Neurology. 2015;85(9):829–30. https://doi.org/10.1212/WNL.0000000000001892. Neuropathologic findings in acute parenchymal brain lesion of Newuo-sweet disease.
Kokubo Y, Kuzuhara S, Isoda K, Sato K, Kawada N, Narita Y. Neuro-Sweet disease: report of the first autopsy case. J Neurol Neurosurg Psychiatry. 2007;78(9):997–1000. https://doi.org/10.1136/jnnp.2006.105650.
Akiba C, Esaki T, Ando M, Furuya T, Noda K, Nakao Y, et al. Possible neuro-Sweet disease mimicking brain tumor in the medulla oblongata--case report. Neurol Med Chir (Tokyo). 2011;51(2):140–3.
Nakanishi E, Sawamura M, Maruhama S, Yamada H, Kim G, Harada K. Neuro-neutrophilic disease suspected by human leukocyte antigen (HLA) typing and brain biopsy: a case report. Rinsho Shinkeigaku. 2015;55(1):13–7. https://doi.org/10.5692/clinicalneurol.55.13.
Magro CM, Momtahen S, Nguyen GH, Wang X. Histiocytoid Sweet’s syndrome: a localized cutaneous proliferation of macrophages frequently associated with chronic myeloproliferative disease. Eur J Dermatol. 2015;25(4):335–41. https://doi.org/10.1684/ejd.2015.2586.
Delluc A, Limal N, Puechal X, Frances C, Piette JC, Cacoub P. Efficacy of anakinra, an IL1 receptor antagonist, in refractory Sweet syndrome. Ann Rheum Dis. 2008;67(2):278–9. https://doi.org/10.1136/ard.2006.068254.
Prat L, Bouaziz JD, Wallach D, Vignon-Pennamen MD, Bagot M. Neutrophilic dermatoses as systemic diseases. Clin Dermatol. 2014;32(3):376–88. https://doi.org/10.1016/j.clindermatol.2013.11.004.
Seminario-Vidal L, Guerrero C, Sami N. Refractory Sweet’s syndrome successfully treated with rituximab. JAAD Case Rep. 2015;1(3):123–5. https://doi.org/10.1016/j.jdcr.2015.03.002.
Keidel S, McColl A, Edmonds S. Sweet’s syndrome after adalimumab therapy for refractory relapsing polychondritis. BMJ Case Rep. 2011;2011. https://doi.org/10.1136/bcr.10.2011.4935.
• Maalouf D, Battistella M, Bouaziz JD. Neutrophilic dermatosis: disease mechanism and treatment. Curr Opin Hematol. 2015;22(1):23–9. https://doi.org/10.1097/MOH.0000000000000100. Review of therapeutic options, including anti-IL-1 therapy for neutrophilic dermatoses.
• Pinho J, Rocha J, Sousa F, Macedo C, Soares-Fernandes J, Cerqueira J, et al. Localized scleroderma en coup de sabre in the neurology clinic. Mult Scler Relat Disord. 2016;8:96–8. https://doi.org/10.1016/j.msard.2016.05.013. Five detailed neurological cases with MRIs findings.
Kraus V, Lawson EF, von Scheven E, Tihan T, Garza J, Nathan RG, et al. Atypical cases of scleroderma en coup de sabre. J Child Neurol. 2014;29(5):698–703. https://doi.org/10.1177/0883073813520494.
Rongioletti F, Ferreli C, Atzori L, Bottoni U, Soda G. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153(2):208–15. https://doi.org/10.23736/S0392-0488.18.05922-9.
Gordon JK, Martyanov V, Magro C, Wildman HF, Wood TA, Huang WT, et al. Nilotinib (Tasigna) in the treatment of early diffuse systemic sclerosis: an open-label, pilot clinical trial. Arthritis Res Ther. 2015;17:213. https://doi.org/10.1186/s13075-015-0721-3.
Gordon J, Udeh U, Doobay K, Magro C, Wildman H, Davids M, et al. Imatinib mesylate (Gleevec) in the treatment of diffuse cutaneous systemic sclerosis: results of a 24-month open label, extension phase, single-centre trial. Clin Exp Rheumatol. 2014;32(6 Suppl 86):S-189–93.
Karaca NE, Aksu G, Karaca E, Tuzun F, Gunes AT, Ozkinay F, et al. Progressive morphea of early childhood tracing Blaschko’s lines on the face: involvement of X chromosome monosomy in pathogenesis and clinical prognosis. Int J Dermatol. 2011;50(11):1406–10. https://doi.org/10.1111/j.1365-4632.2011.04900.x.
Leitenberger JJ, Cayce RL, Haley RW, Adams-Huet B, Bergstresser PR, Jacobe HT. Distinct autoimmune syndromes in morphea: a review of 245 adult and pediatric cases. Arch Dermatol. 2009;145(5):545–50. https://doi.org/10.1001/archdermatol.2009.79.
Jacobe H, Ahn C, Arnett FC, Reveille JD. Major histocompatibility complex class I and class II alleles may confer susceptibility to or protection against morphea: findings from the Morphea in adults and children cohort. Arthritis Rheumatol. 2014;66(11):3170–7. https://doi.org/10.1002/art.38814.
Pham AK, Srivastava B, Deng A. Pregnancy-associated morphea: a case report and literature review. Dermatol Online J. 2017;23(1).
Christianson HB, Dorsey CS, Kierland RR, O'Leary PA. Localized scleroderma; a clinical study of two hundred thirty-five cases. AMA Arch Derm. 1956;74(6):629–39.
Khamaganova I. Localized scleroderma: predisposing and triggering factors. Open Dermatol J 2017;11:1–11. https://doi.org/10.2174/1874372201711010001.
Fruchter R, Kurtzman DJB, Mazori DR, Wright NA, Patel M, Vleugels RA, et al. Characteristics and treatment of postirradiation morphea: a retrospective multicenter analysis. J Am Acad Dermatol. 2017;76(1):19–21. https://doi.org/10.1016/j.jaad.2016.08.059.
De Somer L, Morren MA, Muller PC, Despontin K, Jansen K, Lagae L, et al. Overlap between linear scleroderma, progressive facial hemiatrophy and immune-inflammatory encephalitis in a paediatric cohort. Eur J Pediatr. 2015;174(9):1247–54. https://doi.org/10.1007/s00431-015-2532-6.
Zulian F, Athreya BH, Laxer R, Nelson AM, Feitosa de Oliveira SK, Punaro MG, et al. Juvenile localized scleroderma: clinical and epidemiological features in 750 children. An international study. Rheumatology (Oxford). 2006;45(5):614–20. https://doi.org/10.1093/rheumatology/kei251.
Magro CM, Ross P, Marsh CB, Allen JN, Liff D, Knight DA, et al. The role of anti-endothelial cell antibody-mediated microvascular injury in the evolution of pulmonary fibrosis in the setting of collagen vascular disease. Am J Clin Pathol. 2007;127(2):237–47. https://doi.org/10.1309/CNQDMHLH2WGKL32T.
Wusirika R, Ferri C, Marin M, Knight DA, Waldman WJ, Ross P Jr, et al. The assessment of anti-endothelial cell antibodies in scleroderma-associated pulmonary fibrosis. A study of indirect immunofluorescent and western blot analysis in 49 patients with scleroderma. Am J Clin Pathol. 2003;120(4):596–606. https://doi.org/10.1309/8HVC-MJMY-NPUQ-PBD2.
Peterson LS, Nelson AM, Su WP. Classification of morphea (localized scleroderma). Mayo Clin Proc. 1995;70(11):1068–76. https://doi.org/10.1016/S0025-6196(11)64442-X.
• Wong M, Phillips CD, Hagiwara M, Shatzkes DR. Parry Romberg syndrome: 7 cases and literature review. AJNR Am J Neuroradiol. 2015;36(7):1355–61. https://doi.org/10.3174/ajnr.A4297. Seven neurological patient cases with relevant neuroimaging.
Fett N, Werth VP. Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2011;64(2):217–28; quiz 29-30. https://doi.org/10.1016/j.jaad.2010.05.045.
Kister I, Inglese M, Laxer RM, Herbert J. Neurologic manifestations of localized scleroderma: a case report and literature review. Neurology. 2008;71(19):1538–45. https://doi.org/10.1212/01.wnl.0000334474.88923.e3.
Sommer A, Gambichler T, Bacharach-Buhles M, von Rothenburg T, Altmeyer P, Kreuter A. Clinical and serological characteristics of progressive facial hemiatrophy: a case series of 12 patients. J Am Acad Dermatol. 2006;54(2):227–33. https://doi.org/10.1016/j.jaad.2005.10.020.
Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: a retrospective review of 54 patients. J Am Acad Dermatol. 2007;56(2):257–63. https://doi.org/10.1016/j.jaad.2006.10.959.
Chiu YE, Vora S, Kwon EK, Maheshwari M. A significant proportion of children with morphea en coup de sabre and Parry-Romberg syndrome have neuroimaging findings. Pediatr Dermatol. 2012;29(6):738–48. https://doi.org/10.1111/pde.12001.
Christen-Zaech S, Hakim MD, Afsar FS, Paller AS. Pediatric morphea (localized scleroderma): review of 136 patients. J Am Acad Dermatol. 2008;59(3):385–96. https://doi.org/10.1016/j.jaad.2008.05.005.
• Mansour M, Liy Wong C, Zulian F, Li S, Morishita K, Yeh EA, et al. Natural history and extracutaneous involvement of congenital morphea: Multicenter retrospective cohort study and literature review. Pediatr Dermatol. 2018;35(6):761–8. https://doi.org/10.1111/pde.13605. Different types of morphea found in neonates (congenital morphea) are reviewed including clinical characteristics, extracutaneous manifestations and laboratory markers.
Amaral TN, Peres FA, Lapa AT, Marques-Neto JF, Appenzeller S. Neurologic involvement in scleroderma: a systematic review. Semin Arthritis Rheum. 2013;43(3):335–47. https://doi.org/10.1016/j.semarthrit.2013.05.002.
Carreno M, Donaire A, Barcelo MI, Rumia J, Falip M, Agudo R, et al. Parry Romberg syndrome and linear scleroderma in coup de sabre mimicking Rasmussen encephalitis. Neurology. 2007;68(16):1308–10. https://doi.org/10.1212/01.wnl.0000259523.09001.7a.
Doolittle DA, Lehman VT, Schwartz KM, Wong-Kisiel LC, Lehman JS, Tollefson MM. CNS imaging findings associated with Parry-Romberg syndrome and en coup de sabre: correlation to dermatologic and neurologic abnormalities. Neuroradiology. 2015;57(1):21–34. https://doi.org/10.1007/s00234-014-1448-6.
• Maloney E, Menashe SJ, Iyer RS, Ringold S, Chakraborty AK, Ishak GE. The central nervous system manifestations of localized craniofacial scleroderma: a study of 10 cases and literature review. Pediatr Radiol. 2018;48(11):1642–54. https://doi.org/10.1007/s00247-018-4177-x. CNS manifestations of craniofacial scleroderma and impressive imaging findings, including single case of PET/CT in a morphea patient.
Fain ET, Mannion M, Pope E, Young DW, Laxer RM, Cron RQ. Brain cavernomas associated with en coup de sabre linear scleroderma: two case reports. Pediatr Rheumatol Online J. 2011;9:18. https://doi.org/10.1186/1546-0096-9-18.
Stone J, Franks AJ, Guthrie JA, Johnson MH. Scleroderma “en coup de sabre”: pathological evidence of intracerebral inflammation. J Neurol Neurosurg Psychiatry. 2001;70(3):382–5.
English SW, Ho ML, Tollefson MM, Wong-Kisiel LC. Focal epilepsy in a teenager with facial atrophy and hair loss. Semin Pediatr Neurol. 2018;26:68–73. https://doi.org/10.1016/j.spen.2017.03.009.
Rimoin L, Arbiser J. Improvement of “en coup de sabre” morphea and associated headaches with botulinum toxin injections. Dermatol Surg. 2016;42(10):1216–9. https://doi.org/10.1097/DSS.0000000000000812.
Infante-Valenzuela A, Camara-Lemarroy CR, Delgado-Garcia G, Gil-Valdez AH, Marfil-Rivera A. Steroid-responsive headache in scleroderma en coup de sabre. Acta Neurol Belg. 2017;117(1):405–7. https://doi.org/10.1007/s13760-016-0673-7.
Polcari I, Moon A, Mathes EF, Gilmore ES, Paller AS. Headaches as a presenting symptom of linear morphea en coup de sabre. Pediatrics. 2014;134(6):e1715–9. https://doi.org/10.1542/peds.2014-0019.
Camacho-Velasquez JL. Nummular headache associated with linear scleroderma. Headache. 2016;56(9):1492–3. https://doi.org/10.1111/head.12894.
Lenassi E, Vassallo G, Kehdi E, Chieng AS, Ashworth JL. Craniofacial linear scleroderma associated with retinal telangiectasia and exudative retinal detachment. J AAPOS. 2017;21(3):251–4. https://doi.org/10.1016/j.jaapos.2016.12.004.
Walker RH, Fink JK. Morphea and Parry-Romberg syndrome associated with a mixed movement disorder. Parkinsonism Relat Disord. 2013;19(12):1169–70. https://doi.org/10.1016/j.parkreldis.2013.07.025.
Sathornsumetee S, Schanberg L, Rabinovich E, Lewis D Jr, Weisleder P. Parry-Romberg syndrome with fatal brain stem involvement. J Pediatr. 2005;146(3):429–31. https://doi.org/10.1016/j.jpeds.2004.10.026.
Klimiec E, Klimkowicz-Mrowiec A. Mild cognitive impairment as a single sign of brain hemiatrophy in patient with localized scleroderma and Parry-Romberg syndrome. Neurol Neurochir Pol. 2016;50(3):215–8. https://doi.org/10.1016/j.pjnns.2016.02.002.
Miyamoto M, Kinoshita M, Tanaka K, Tanaka M. Recurrent myelitis in localized scleroderma. Clin Neurol Neurosurg. 2014;127:140–2. https://doi.org/10.1016/j.clineuro.2014.10.005.
Kanzato N, Matsuzaki T, Komine Y, Saito M, Saito A, Yoshio T, et al. Localized scleroderma associated with progressing ischemic stroke. J Neurol Sci. 1999;163(1):86–9.
Holland KE, Steffes B, Nocton JJ, Schwabe MJ, Jacobson RD, Drolet BA. Linear scleroderma en coup de sabre with associated neurologic abnormalities. Pediatrics. 2006;117(1):e132–6. https://doi.org/10.1542/peds.2005-0470.
Chung MH, Sum J, Morrell MJ, Horoupian DS. Intracerebral involvement in scleroderma en coup de sabre: report of a case with neuropathologic findings. Ann Neurol. 1995;37(5):679–81. https://doi.org/10.1002/ana.410370519.
Takahashi T, Asano Y, Oka T, Miyagaki T, Tamaki Z, Nonaka S, et al. Scleroderma en coup de sabre with recurrent episodes of brain hemorrhage. J Dermatol. 2016;43(2):203–6. https://doi.org/10.1111/1346-8138.13023.
Franklin ML, Day S. Bilateral choroidal excavation in juvenile localized scleroderma. Retin Cases Brief Rep. 2018;12(1):21–3. https://doi.org/10.1097/ICB.0000000000000381.
Zannin ME, Martini G, Athreya BH, Russo R, Higgins G, Vittadello F, et al. Ocular involvement in children with localised scleroderma: a multi-centre study. Br J Ophthalmol. 2007;91(10):1311–4. https://doi.org/10.1136/bjo.2007.116038.
Laxer RM, Miller S, Pope E. Adie pupil as the initial presentation of localized en coup de sabre scleroderma. J Rheumatol. 2017;44(7):1096–7. https://doi.org/10.3899/jrheum.161442.
Hock LE, Kontzialis M, Szewka AJ. Linear scleroderma en coup de sabre presenting with positional diplopia and enophthalmos. Neurology. 2016;87(16):1741–2. https://doi.org/10.1212/WNL.0000000000003239.
Fledelius HC, Danielsen PL, Ullman S. Ophthalmic findings in linear scleroderma manifesting as facial en coup de sabre. Eye (Lond). 2018;32(11):1688–96. https://doi.org/10.1038/s41433-018-0137-9.
Rosario C, Garelick D, Greenberg G, Chapman J, Shoenfeld Y, Langevitz P. Plaque morphea with neurological involvement-an extraordinary uncommon presentation. Clin Rheumatol. 2015;34(3):597–601. https://doi.org/10.1007/s10067-013-2458-1.
Li SC, Torok KS, Pope E, Dedeoglu F, Hong S, Jacobe HT, et al. Development of consensus treatment plans for juvenile localized scleroderma: a roadmap toward comparative effectiveness studies in juvenile localized scleroderma. Arthritis Care Res (Hoboken). 2012;64(8):1175–85. https://doi.org/10.1002/acr.21687.
Knobler R, Moinzadeh P, Hunzelmann N, Kreuter A, Cozzio A, Mouthon L, et al. European dermatology forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, part 1: localized scleroderma, systemic sclerosis and overlap syndromes. J Eur Acad Dermatol Venereol. 2017;31(9):1401–24. https://doi.org/10.1111/jdv.14458.
Osminina M, Geppe N, Afonina E. Scleroderma “en coup de sabre” with epilepsy and uveitis successfully treated with tocilizumab. Reumatol Clin. 2018. https://doi.org/10.1016/j.reuma.2018.05.001.
Magro CM, Halteh P, Olson LC, Shapiro L, Kister I. Linear scleroderma with brain involvement—Clinicopathologic analysis and response to tocilizumab. Orphanet J Rare Dis. (2019);(in press).
• Lythgoe H, Baildam E, Beresford MW, Cleary G, McCann LJ, Pain CE. Tocilizumab as a potential therapeutic option for children with severe, refractory juvenile localized scleroderma. Rheumatology (Oxford). 2018;57(2):398–401. https://doi.org/10.1093/rheumatology/kex382. Case series of 5 patients with localized scleroderma, demonstrates safety and presumed efficacy of tocilizumab for severe refractory disease.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Asya Wallach reports educational grants from the National MS Society and Biogen. Lee Shapiro reports he served on advisory board for Genentech. Ilya Kister reports he served on advisory boards for Biogen and Genentech and received research support for investigator-initiated grants from Sanofi Genzyme, Biogen, EMD Serono, National MS Society, and Guthy Jackson Charitable Foundation. Cynthia Magro and Andrew Franks each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology of Systemic Diseases
Rights and permissions
About this article
Cite this article
Wallach, A.I., Magro, C.M., Franks, A.G. et al. Protean Neurologic Manifestations of Two Rare Dermatologic Disorders: Sweet Disease and Localized Craniofacial Scleroderma. Curr Neurol Neurosci Rep 19, 11 (2019). https://doi.org/10.1007/s11910-019-0929-8
Published:
DOI: https://doi.org/10.1007/s11910-019-0929-8