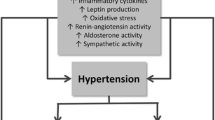
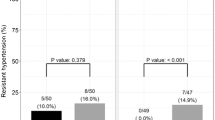
Abstract
Purpose of Review
Obesity increases the risk of hypertension. However, blood pressure decreases before any significant loss of body weight after bariatric surgery. We review the mechanisms of the temporal dissociation between blood pressure and body weight after bariatric surgery.
Recent Findings
Restrictive and bypass bariatric surgery lower blood pressure and plasma leptin levels within days of the procedure in both hypertensive and normotensive morbidly obese patients. Rapidly decreasing plasma leptin levels and minimal loss of body weight point to reduced sympathetic nervous system activity as the underlying mechanism of rapid blood pressure decline after bariatric surgery. After the early rapid decline, blood pressure does not decrease further in patients who, while still obese, experience a steady loss of body weight for the subsequent 12 months. The divergent effects of bariatric surgery on blood pressure and body weight query the role of excess body weight in the pathobiology of the obesity phenotype of hypertension.
Summary
The decrease in blood pressure after bariatric surgery is moderate and independent of body weight. The lack of temporal relationship between blood pressure reduction and loss of body weight for 12 months after sleeve gastrectomy questions the nature of the mechanisms underlying obesity-associated hypertension.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Garrison RJ, Kannel WB, Stokes J, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16(2):235–51. https://doi.org/10.1016/0091-7435(87)90087-9.
Molenaar EA, Hwang SJ, Vasan RS, Grobbee DE, Meigs JB, D’Agostino RB, et al. Burden and rates of treatment and control of cardiovascular disease risk factors in obesity: the Framingham Heart Study. Diabetes Care. 2008;31(7):1367–72. https://doi.org/10.2337/dc07-2413.
Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164(19):2126–34. https://doi.org/10.1001/archinte.164.19.2126.
Pareek M, Bhatt DL. Cardiometabolic risk reduction after metabolic surgery. Curr Opin Cardiol. 2019;34(6):663–72. https://doi.org/10.1097/HCO.0000000000000683.
Kotsis V, Nilsson P, Grassi G, Mancia G, Redon J, Luft F, et al. New developments in the pathogenesis of obesity-induced hypertension. J Hypertens. 2015;33(8):1499–508. https://doi.org/10.1097/HJH.0000000000000645.
Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS, Weidenbacher HJ, et al. Bariatric surgery and long-term durability of weight loss. JAMA Surg. 2016;151(11):1046–55. https://doi.org/10.1001/jamasurg.2016.2317.
Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76. https://doi.org/10.1056/NEJMoa1200225.
Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. https://doi.org/10.1136/bmj.f5934.
Ahmed AR, Rickards G, Coniglio D, Xia Y, Johnson J, Boss T, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg. 2009;19(7):845–9. https://doi.org/10.1007/s11695-008-9671-z.
Pedersen JS, Borup C, Damgaard M, Yatawara VD, Floyd AK, Gadsbøll N, et al. Early 24-hour blood pressure response to Roux-en-Y gastric bypass in obese patients. Scand J Clin Lab Invest. 2017;77(1):53–9. https://doi.org/10.1080/00365513.2016.1258725.
Hawkins DN, Faler BJ, Choi YU, Prasad BM. Time course of blood pressure decrease after bariatric surgery in normotensive and hypertensive patients. Obes Surg. 2018;28(7):1845–51. https://doi.org/10.1007/s11695-017-3091-x.
Samson R, Milligan G, Lewine E, Sindi F, Garagliano J, Fernandez C, et al. Effect of sleeve gastrectomy on hypertension. J Am Soc Hypertens. 2018;12(11):e19–25. https://doi.org/10.1016/j.jash.2018.09.007.
Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res 2015;116(6):991–1006. doi:https://doi.org/10.1161/CIRCRESAHA.116.305697.
DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat Rev Endocrinol. 2014;10(6):364–76. https://doi.org/10.1038/nrendo.2014.44.
Okosun IS, Prewitt TE, Cooper RS. Abdominal obesity in the United States: prevalence and attributable risk of hypertension. J Hum Hypertens. 1999;13(7):425–30. https://doi.org/10.1038/sj.jhh.1000862.
Kröner Florit PT, Corral Hurtado JE, Wijarnpreecha K, Elli EF, Lukens FJ. Bariatric surgery, clinical outcomes, and healthcare burden in Hispanics in the USA. Obes Surg. 2019;29(11):3646–52. https://doi.org/10.1007/s11695-019-04047-4.
Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14(3):160–9. https://doi.org/10.1038/nrgastro.2016.170.
Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377(12):1143–55. https://doi.org/10.1056/NEJMoa1700459.
Ikramuddin S, Korner J, Lee WJ, Thomas AJ, Connett JE, Bantle JP, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA. 2018;319(3):266–78. https://doi.org/10.1001/jama.2017.20813.
Benotti PN, Wood GC, Carey DJ, Mehra VC, Mirshahi T, Lent MR et al. Gastric bypass surgery produces a durable reduction in cardiovascular disease risk factors and reduces the long-term risks of congestive heart failure. J Am Heart Assoc. 2017;6(5). doi:https://doi.org/10.1161/JAHA.116.005126.
Hinojosa MW, Varela JE, Smith BR, Che F, Nguyen NT. Resolution of systemic hypertension after laparoscopic gastric bypass. J Gastrointest Surg. 2009;13(4):793–7. https://doi.org/10.1007/s11605-008-0759-5.
Schiavon CA, Bersch-Ferreira AC, Santucci EV, Oliveira JD, Torreglosa CR, Bueno PT, et al. Effects of bariatric surgery in obese patients with hypertension: the GATEWAY Randomized Trial (Gastric Bypass to Treat Obese Patients With Steady Hypertension). Circulation. 2018;137(11):1132–42. https://doi.org/10.1161/CIRCULATIONAHA.117.032130.
Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment: a position paper of the Obesity Society and the American Society of Hypertension. J Clin Hypertens (Greenwich). 2013;15(1):14–33. https://doi.org/10.1111/jch.12049.
Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation. 2012;126(10):1301–13. https://doi.org/10.1161/CIRCULATIONAHA.111.067264.
Neeland IJ, Poirier P, Després JP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. 2018;137(13):1391–406. https://doi.org/10.1161/CIRCULATIONAHA.117.029617.
Deng T, Lyon CJ, Bergin S, Caligiuri MA, Hsueh WA. Obesity, inflammation, and cancer. Annu Rev Pathol. 2016;11:421–49. https://doi.org/10.1146/annurev-pathol-012615-044359.
Owen JG, Yazdi F, Reisin E. Bariatric surgery and hypertension. Am J Hypertens. 2017;31(1):11–7. https://doi.org/10.1093/ajh/hpx112.
Briones AM, Nguyen Dinh Cat A, Callera GE, Yogi A, Burger D, He Y et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59(5):1069–1078. doi:https://doi.org/10.1161/HYPERTENSIONAHA.111.190223.
Alvarez GE, Beske SD, Ballard TP, Davy KP. Sympathetic neural activation in visceral obesity. Circulation. 2002;106(20):2533–6. https://doi.org/10.1161/01.cir.0000041244.79165.25.
Lim K, Barzel B, Burke SL, Armitage JA, Head GA. Origin of aberrant blood pressure and sympathetic regulation in diet-induced obesity. Hypertension. 2016;68(2):491–500. https://doi.org/10.1161/HYPERTENSIONAHA.116.07461.
Lim K, Burke SL, Head GA. Obesity-related hypertension and the role of insulin and leptin in high-fat-fed rabbits. Hypertension. 2013;61(3):628–34. https://doi.org/10.1161/HYPERTENSIONAHA.111.00705.
Lim K, Jackson KL, Sata Y, Head GA. Factors responsible for obesity-related hypertension. Curr Hypertens Rep. 2017;19(7):53. https://doi.org/10.1007/s11906-017-0750-1.
Seravalle G, Colombo M, Perego P, Giardini V, Volpe M, Dell’Oro R, et al. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension. 2014;64(2):431–7. https://doi.org/10.1161/HYPERTENSIONAHA.113.02988.
Shankar A, Xiao J. Positive relationship between plasma leptin level and hypertension. Hypertension. 2010;56(4):623–8. https://doi.org/10.1161/HYPERTENSIONAHA.109.148213.
Stocker SD, Kinsman BJ, Sved AF. Recent advances in neurogenic hypertension: dietary salt, obesity, and inflammation. Hypertension. 2017. https://doi.org/10.1161/HYPERTENSIONAHA.117.08936.
Bell BB, Rahmouni K. Leptin as a mediator of obesity-induced hypertension. Curr Obes Rep. 2016;5(4):397–404. https://doi.org/10.1007/s13679-016-0231-x.
Faulkner JL, Belin de Chantemèle EJ. Sex differences in mechanisms of hypertension associated with obesity. Hypertension. 2018;71(1):15–21. https://doi.org/10.1161/HYPERTENSIONAHA.117.09980.
Bochud M, Marquant F, Marques-Vidal PM, Vollenweider P, Beckmann JS, Mooser V, et al. Association between C-reactive protein and adiposity in women. J Clin Endocrinol Metab. 2009;94(10):3969–77. https://doi.org/10.1210/jc.2008-2428.
Browning LM, Krebs JD, Magee EC, Frühbeck G, Jebb SA. Circulating markers of inflammation and their link to indices of adiposity. Obes Facts. 2008;1(5):259–65. https://doi.org/10.1159/000169832.
de Gusmão Correia ML. Adiposity and hypertension: the inflammatory link. J Hypertens. 2010;28(7):1377–9. https://doi.org/10.1097/HJH.0b013e32833b7862.
Madec S, Chiarugi M, Santini E, Rossi C, Miccoli P, Ferrannini E, et al. Pattern of expression of inflammatory markers in adipose tissue of untreated hypertensive patients. J Hypertens. 2010;28(7):1459–65. https://doi.org/10.1097/hjh.0b013e3283388871.
Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. https://doi.org/10.1038/nri2921.
Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, et al. Inflammation, immunity, and hypertension. Hypertension. 2011;57(2):132–40. https://doi.org/10.1161/HYPERTENSIONAHA.110.163576.
Kneedler SC, Phillips LE, Hudson KR, Beckman KM, Lopez Gelston CA, Rutkowski JM, et al. Renal inflammation and injury are associated with lymphangiogenesis in hypertension. Am J Physiol Renal Physiol. 2017;312(5):F861–F9. https://doi.org/10.1152/ajprenal.00679.2016.
McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res. 2015;116(6):1022–33. https://doi.org/10.1161/CIRCRESAHA.116.303697.
Higaki A, Caillon A, Paradis P, Schiffrin EL. Innate and innate-like immune system in hypertension and vascular injury. Curr Hypertens Rep. 2019;21(1):4. https://doi.org/10.1007/s11906-019-0907-1.
Itani HA, McMaster WG, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68(1):123–32. https://doi.org/10.1161/HYPERTENSIONAHA.116.07237.
Miller IM, Ellervik C, Yazdanyar S, Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 2013;69(6):1014–24. https://doi.org/10.1016/j.jaad.2013.06.053.
Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford). 2008;47(9):1286–98. https://doi.org/10.1093/rheumatology/ken159.
Rakotoarivelo V, Lacraz G, Mayhue M, Brown C, Rottembourg D, Fradette J, et al. Inflammatory cytokine profiles in visceral and subcutaneous adipose tissues of obese patients undergoing bariatric surgery reveal lack of correlation with obesity or diabetes. EBioMedicine. 2018;30:237–47. https://doi.org/10.1016/j.ebiom.2018.03.004.
Vinolas H, Barnetche T, Ferrandi G, Monsaingeon-Henry M, Pupier E, Collet D, et al. Oral hydration, food intake, and nutritional status before and after bariatric surgery. Obes Surg. 2019;29(9):2896–903. https://doi.org/10.1007/s11695-019-03928-y.
Docherty NG, Fändriks L, le Roux CW, Hallersund P, Werling M. Urinary sodium excretion after gastric bypass surgery. Surg Obes Relat Dis. 2017;13(9):1506–14. https://doi.org/10.1016/j.soard.2017.04.002.
Hallersund P, Sjöström L, Olbers T, Lönroth H, Jacobson P, Wallenius V, et al. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis - long term results from the Swedish obese subjects (SOS) study. PLoS One. 2012;7(11):e49696. https://doi.org/10.1371/journal.pone.0049696.
Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33(7):1438–42. https://doi.org/10.2337/dc09-2107.
Halpern B, Mancini MC. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI lower than 35 kg/m. Obes Rev. 2019;20(5):633–47. https://doi.org/10.1111/obr.12837.
Nicoll R, Henein MY. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int J Mol Sci. 2018;19(3). doi:https://doi.org/10.3390/ijms19030751.
•• Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102(5):519–28. https://doi.org/10.1161/CIRCRESAHA.107.168369The paper underlines the beneficial vascular effect of calorie restriction that attenuates vascular generation of reactive oxygen species through the Nrf2/ARE pathway and thereby enhances nitric oxide bioavailability.
Sasaki S, Higashi Y, Nakagawa K, Kimura M, Noma K, Hara K, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15(4 Pt 1):302–9. https://doi.org/10.1016/s0895-7061(01)02322-6.
Raitakari M, Ilvonen T, Ahotupa M, Lehtimäki T, Harmoinen A, Suominen P, et al. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24(1):124–8. https://doi.org/10.1161/01.ATV.0000109749.11042.7c.
Zhang H, Pu Y, Chen J, Tong W, Cui Y, Sun F, et al. Gastrointestinal intervention ameliorates high blood pressure through antagonizing overdrive of the sympathetic nerve in hypertensive patients and rats. J Am Heart Assoc. 2014;3(5):e000929. https://doi.org/10.1161/JAHA.114.000929.
•• Wewer Albrechtsen NJ, Geyer PE, Doll S, Treit PV, Bojsen-Møller KN, Martinussen C, et al. Plasma proteome profiling reveals dynamics of inflammatory and lipid homeostasis markers after Roux-En-Y gastric bypass surgery. Cell Syst. 2018;7(6):601–12.e3. https://doi.org/10.1016/j.cels.2018.10.012The authors investigated the effect of Roux-en-Y gastric bypass (RYGB) on the plasma proteome using a mass spectrometric strategy. The changes in inflammation-associated proteins after RYGB are comparable to that previously reported after caloric restriction-induced weight loss.
Richards EM, Pepine CJ, Raizada MK, Kim S. The gut, its microbiome, and hypertension. Curr Hypertens Rep. 2017;19(4):36. https://doi.org/10.1007/s11906-017-0734-1.
Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21(8):63. https://doi.org/10.1007/s11906-019-0964-5.
McGavigan AK, Henseler ZM, Garibay D, Butler SD, Jayasinghe S, Ley RE, et al. Vertical sleeve gastrectomy reduces blood pressure and hypothalamic endoplasmic reticulum stress in mice. Dis Model Mech. 2017;10(3):235–43. https://doi.org/10.1242/dmm.027474.
Larraufie P, Roberts GP, McGavigan AK, Kay RG, Li J, Leiter A, et al. Important role of the GLP-1 axis for glucose homeostasis after bariatric surgery. Cell Rep. 2019;26(6):1399–408.e6. https://doi.org/10.1016/j.celrep.2019.01.047.
Garibay D, Lou J, Lee SA, Zaborska KE, Weissman MH, Sloma E, et al. β cell GLP-1R signaling alters α cell proglucagon processing after vertical sleeve gastrectomy in mice. Cell Rep. 2018;23(4):967–73. https://doi.org/10.1016/j.celrep.2018.03.120.
Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. J Clin Invest. 2011;121(6):2087–93. https://doi.org/10.1172/JCI45888.
Mark AL, Agassandian K, Morgan DA, Liu X, Cassell MD, Rahmouni K. Leptin signaling in the nucleus tractus solitarii increases sympathetic nerve activity to the kidney. Hypertension. 2009;53(2):375–80. https://doi.org/10.1161/HYPERTENSIONAHA.108.124255.
Hillebrand JJG, Geary N. Do leptin and insulin signal adiposity? Forum Nutr. 2010;63:111–22. https://doi.org/10.1159/000264399.
Sinha MK, Ohannesian JP, Heiman ML, Kriauciunas A, Stephens TW, Magosin S, et al. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J Clin Invest. 1996;97(5):1344–7. https://doi.org/10.1172/JCI118551.
Cho YM, Kim MS, Shin CS, Park DJ, Park KS, Yang HK, et al. Dynamic change in plasma leptin level during the perioperative period. Horm Res. 2003;59(2):100–4. https://doi.org/10.1159/000068579.
Nijhuis J, van Dielen FM, Buurman WA, Greve JW. Ghrelin, leptin and insulin levels after restrictive surgery: a 2-year follow-up study. Obes Surg. 2004;14(6):783–7. https://doi.org/10.1381/0960892041590980.
Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbély Y, Beglinger C. Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy--a prospective randomized trial. Surg Obes Relat Dis. 2011;7(5):561–8. https://doi.org/10.1016/j.soard.2011.01.044.
Stephens JW, Min T, Dunseath G, Churm R, Barry JD, Prior SL. Temporal effects of laparoscopic sleeve gastrectomy on adipokines, inflammation, and oxidative stress in patients with impaired glucose homeostasis. Surg Obes Relat Dis. 2019;15(12):2011–7. https://doi.org/10.1016/j.soard.2019.04.006.
Seridi L, Leo GC, Dohm GL, Pories WJ, Lenhard J. Time course metabolome of Roux-en-Y gastric bypass confirms correlation between leptin, body weight and the microbiome. PLoS One. 2018;13(5):e0198156. https://doi.org/10.1371/journal.pone.0198156.
Lindegaard KK, Jorgensen NB, Just R, Heegaard PM, Madsbad S. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr. 2015;7:12. https://doi.org/10.1186/s13098-015-0012-9.
Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, et al. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr. 2011;35(2):169–80. https://doi.org/10.1177/0148607110381403.
Rodríguez A, Becerril S, Valentí V, Moncada R, Méndez-Giménez L, Ramírez B, et al. Short-term effects of sleeve gastrectomy and caloric restriction on blood pressure in diet-induced obese rats. Obes Surg. 2012;22(9):1481–90. https://doi.org/10.1007/s11695-012-0702-4.
Kain ZN, Zimolo Z, Heninger G. Leptin and the perioperative neuroendocrinological stress response. J Clin Endocrinol Metab. 1999;84(7):2438–42. https://doi.org/10.1210/jcem.84.7.5850.
Stratton RJ, Dewit O, Crowe E, Jennings G, Villar RN, Elia M. Plasma leptin, energy intake and hunger following total hip replacement surgery. Clin Sci (Lond). 1997;93(2):113–7. https://doi.org/10.1042/cs0930113.
Montalbán C, Del Moral I, García-Unzueta MT, Villanueva MA, Amado JA. Perioperative response of leptin and the tumor necrosis factor alpha system in morbidly obese patients. Influence of cortisol inhibition by etomidate. Acta Anaesthesiol Scand. 2001;45(2):207–12. https://doi.org/10.1034/j.1399-6576.2001.450212.x.
Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, et al. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. 1996;45(11):1511–5. https://doi.org/10.2337/diab.45.11.1511.
Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source of leptin. Nature. 1998;394(6695):790–3. https://doi.org/10.1038/29547.
Galletti F, D’Elia L, Barba G, Siani A, Cappuccio FP, Farinaro E, et al. High-circulating leptin levels are associated with greater risk of hypertension in men independently of body mass and insulin resistance: results of an eight-year follow-up study. J Clin Endocrinol Metab. 2008;93(10):3922–6. https://doi.org/10.1210/jc.2008-1280.
Asferg C, Møgelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, et al. Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. Am J Hypertens. 2010;23(3):327–33. https://doi.org/10.1038/ajh.2009.244.
Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997;10(10 Pt 1):1171–4. https://doi.org/10.1016/s0895-7061(97)00310-5.
Simonds SE, Pryor JT, Cowley MA. Does leptin cause an increase in blood pressure in animals and humans? Curr Opin Nephrol Hypertens. 2017;26(1):20–5. https://doi.org/10.1097/MNH.0000000000000287.
Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol. 2013;305(6):R566–81. https://doi.org/10.1152/ajpregu.00180.2013.
•• Becerril S, Rodríguez A, Catalán V, Ramírez B, Unamuno X, Portincasa P, et al. Functional relationship between leptin and nitric oxide in metabolism. Nutrients. 2019;11(9). https://doi.org/10.3390/nu11092129Leptin exerts multiple effects on thermogenesis, angiogenesis, hematopoiesis, and immune function in addition to blood pressure modulation. Nitric oxide also exerts multiple biological effects on energy balance, immune response, blood pressure, and reproduction. The review describes the complex interaction between leptin and nitric oxide in these biological processes.
Bełtowski J, Jochem J, Wójcicka G, Zwirska-Korczala K. Influence of intravenously administered leptin on nitric oxide production, renal hemodynamics and renal function in the rat. Regul Pept. 2004;120(1–3):59–67. https://doi.org/10.1016/j.regpep.2004.02.012.
Avogaro A, de Kreutzenberg SV. Mechanisms of endothelial dysfunction in obesity. Clin Chim Acta. 2005;360(1–2):9–26. https://doi.org/10.1016/j.cccn.2005.04.020.
Campia U, Tesauro M, Cardillo C. Human obesity and endothelium-dependent responsiveness. Br J Pharmacol. 2012;165(3):561–73. https://doi.org/10.1111/j.1476-5381.2011.01661.x.
•• Grassi G, Biffi A, Seravalle G, Trevano FQ, Dell’Oro R, Corrao G, et al. Sympathetic neural overdrive in the obese and overweight state. Hypertension. 2019;74(2):349–58. https://doi.org/10.1161/HYPERTENSIONAHA.119.12885Muscle sympathetic nerve traffic (MSNA) was evaluated in 1438 obese or overweight subjects without hypertension or metabolic syndrome who were recruited in 45 microneurographic studies. MSNA was significantly greater in obese and overweight subjects than in normal weight subjects and also significantly greater in obese than overweight subjects. Importantly, MSNA was directly related to metabolic parameters and not to plasma norepinephrine or heart rate.
Esler M, Straznicky N, Eikelis N, Masuo K, Lambert G, Lambert E. Mechanisms of sympathetic activation in obesity-related hypertension. Hypertension. 2006;48(5):787–96. https://doi.org/10.1161/01.HYP.0000242642.42177.49.
Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287(1):H414–8. https://doi.org/10.1152/ajpheart.01046.2003.
Pontiroli AE, Merlotti C, Veronelli A, Lombardi F. Effect of weight loss on sympatho-vagal balance in subjects with grade-3 obesity: restrictive surgery versus hypocaloric diet. Acta Diabetol. 2013;50(6):843–50. https://doi.org/10.1007/s00592-013-0454-1.
Haynes WG. Interaction between leptin and sympathetic nervous system in hypertension. Curr Hypertens Rep. 2000;2(3):311–8. https://doi.org/10.1007/s11906-000-0015-1.
Rahmouni K. Cardiovascular regulation by the arcuate nucleus of the hypothalamus: neurocircuitry and signaling systems. Hypertension. 2016;67(6):1064–71. https://doi.org/10.1161/HYPERTENSIONAHA.115.06425.
Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, et al. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension. 2012;60(1):163–71. https://doi.org/10.1161/HYPERTENSIONAHA.111.190413.
Prior LJ, Eikelis N, Armitage JA, Davern PJ, Burke SL, Montani JP, et al. Exposure to a high-fat diet alters leptin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2010;55(4):862–8. https://doi.org/10.1161/HYPERTENSIONAHA.109.141119.
Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res. 2013;23(1):1–7. https://doi.org/10.1007/s10286-012-0168-4.
Shi Z, Li B, Brooks VL. Role of the paraventricular nucleus of the hypothalamus in the sympathoexcitatory effects of leptin. Hypertension. 2015;66(5):1034–41. https://doi.org/10.1161/HYPERTENSIONAHA.115.06017.
Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61(3):737–44. https://doi.org/10.1161/HYPERTENSIONAHA.111.00405.
Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, et al. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res. 2011;108(7):808–12. https://doi.org/10.1161/CIRCRESAHA.111.240226.
•• Shin MK, Eraso CC, Mu YP, Gu C, Yeung BHY, Kim LJ, et al. Leptin induces hypertension acting on transient receptor potential melastatin 7 channel in the carotid body. Circ Res. 2019;125(11):989–1002. https://doi.org/10.1161/CIRCRESAHA.119.315338Leptin increases blood pressure through its action on the dorsomedial hypothalamus. This study in mice elucidates the peripheral action of leptin on blood pressure. Leptin increases blood pressure by acting on transient receptor melastatin 7 in the carotid body.
Head GA, Lim K, Barzel B, Burke SL, Davern PJ. Central nervous system dysfunction in obesity-induced hypertension. Curr Hypertens Rep. 2014;16(9):466. https://doi.org/10.1007/s11906-014-0466-4.
Esler M, Lambert G, Schlaich M, Dixon J, Sari CI, Lambert E. Obesity paradox in hypertension: is this because sympathetic activation in obesity-hypertension takes a benign form? Hypertension. 2018;71(1):22–33. https://doi.org/10.1161/HYPERTENSIONAHA.117.09790.
Masuo K, Mikami H, Ogihara T, Tuck ML. Differences in mechanisms between weight loss-sensitive and -resistant blood pressure reduction in obese subjects. Hypertens Res. 2001;24(4):371–6. https://doi.org/10.1291/hypres.24.371.
Masuo K, Mikami H, Ogihara T, Tuck ML. Weight gain-induced blood pressure elevation. Hypertension. 2000;35(5):1135–40. https://doi.org/10.1161/01.hyp.35.5.1135.
•• Grassi G, Pisano A, Bolignano D, Seravalle G, D’Arrigo G, Quarti-Trevano F, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta-analyses. Hypertension. 2018;72(2):483–91. https://doi.org/10.1161/HYPERTENSIONAHA.118.11038This meta-analysis of 63 studies involving 1216 patients unequivocally demonstrates that muscle sympathetic nerve activity (MSNA) is increased in moderate to severe essential hypertension. Importantly, MSNA is closely related to clinic and 24-h ambulatory blood pressure and cardiovascular damage. Borderline hypertension and pre-hypertension are also associated with increased MSNA.
Głuszewska A, Gryglewska B, Gąsowski J, Bilo G, Zarzycki B, Dzieża-Grudnik A, et al. Reduction of 24-h blood pressure variability in extreme obese patients 10 days and 6 months after bariatric surgery depending on pre-existing hypertension. Eur J Intern Med. 2019;60:39–45. https://doi.org/10.1016/j.ejim.2018.10.022.
Ernsberger P, Nelson DO. Refeeding hypertension in dietary obesity. Am J Phys. 1988;254(1 Pt 2):R47–55. https://doi.org/10.1152/ajpregu.1988.254.1.R47.
van de Borne P, Watrin I, Bouquegneau M, Gilles A, Houben JJ, Fery F, et al. Ambulatory blood pressure and neuroendocrine control after diet-assisted gastric restrictive surgery. J Hypertens. 2000;18(3):301–6. https://doi.org/10.1097/00004872-200018030-00010.
Staessen J, Fagard R, Lijnen P, Thijs L, van Hoof R, Amery A. Reference values for ambulatory blood pressure: a meta-analysis. J Hypertens Suppl. 1990;8(6):S57–64.
Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003;42(5):878–84. https://doi.org/10.1161/01.HYP.0000094221.86888.AE.
Aucott L, Poobalan A, Smith WC, Avenell A, Jung R, Broom J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005;45(6):1035–41. https://doi.org/10.1161/01.HYP.0000165680.59733.d4.
Sjöström CD, Peltonen M, Wedel H, Sjöström L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension. 2000;36(1):20–5. https://doi.org/10.1161/01.hyp.36.1.20.
Sjöström CD, Peltonen M, Sjöström L. Blood pressure and pulse pressure during long-term weight loss in the obese: the Swedish Obese Subjects (SOS) Intervention Study. Obes Res. 2001;9(3):188–95. https://doi.org/10.1038/oby.2001.20.
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386(9997):964–73. https://doi.org/10.1016/S0140-6736(15)00075-6.
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376(7):641–51. https://doi.org/10.1056/NEJMoa1600869.
•• Cheng X, Zhang D, Luo S, Qin S. Effect of catheter-based renal denervation on uncontrolled hypertension: a systematic review and meta-analysis. Mayo Clin Proc. 2019;94(9):1695–706. https://doi.org/10.1016/j.mayocp.2019.07.005The article provides an up-to-date systematic review and meta-analysis of the efficacy and safety of catheter-based renal denervation (RDN) for the treatment of uncontrolled hypertension. Pooled analyses of 12 randomized controlled trials reveal that RDN yields significant reductions in ambulatory and office systolic blood pressure compared with controls: mean difference of − 4.02 and − 8.93 mmHg, respectively.
Hult M, Bonn SE, Brandt L, Wirén M, Lagerros YT. Women’s satisfaction with and reasons to seek bariatric surgery-a prospective study in Sweden with 1-year follow-up. Obes Surg. 2019;29(7):2059–70. https://doi.org/10.1007/s11695-019-03834-3.
Pearl RL, Wadden TA, Walton K, Allison KC, Tronieri JS, Williams NN. Health and appearance: factors motivating the decision to seek bariatric surgery. Surg Obes Relat Dis. 2019;15(4):636–42. https://doi.org/10.1016/j.soard.2019.01.015.
Cohen JB, Cohen DL. Integrating out-of-office blood pressure in the diagnosis and management of hypertension. Curr Cardiol Rep. 2016;18(11):112. https://doi.org/10.1007/s11886-016-0780-3.
Foley EF, Benotti PN, Borlase BC, Hollingshead J, Blackburn GL. Impact of gastric restrictive surgery on hypertension in the morbidly obese. Am J Surg. 1992;163(3):294–7. https://doi.org/10.1016/0002-9610(92)90005-c.
Carson JL, Ruddy ME, Duff AE, Holmes NJ, Cody RP, Brolin RE. The effect of gastric bypass surgery on hypertension in morbidly obese patients. Arch Intern Med. 1994;154(2):193–200.
Ricci C, Gaeta M, Rausa E, Asti E, Bandera F, Bonavina L. Long-term effects of bariatric surgery on type II diabetes, hypertension and hyperlipidemia: a meta-analysis and meta-regression study with 5-year follow-up. Obes Surg. 2015;25(3):397–405. https://doi.org/10.1007/s11695-014-1442-4.
Benaiges D, Sagué M, Flores-Le Roux JA, Pedro-Botet J, Ramón JM, Villatoro M, et al. Predictors of hypertension remission and recurrence after bariatric surgery. Am J Hypertens. 2016;29(5):653–9. https://doi.org/10.1093/ajh/hpv153.
Dall’Asta C, Vedani P, Manunta P, Pizzocri P, Marchi M, Paganelli M, et al. Effect of weight loss through laparoscopic gastric banding on blood pressure, plasma renin activity and aldosterone levels in morbid obesity. Nutr Metab Cardiovasc Dis. 2009;19(2):110–4. https://doi.org/10.1016/j.numecd.2008.06.001.
Petroni R, Di Mauro M, Altorio SF, Romano S, Petroni A, Penco M. The role of bariatric surgery for improvement of hypertension in obese patients: a retrospective study. J Cardiovasc Med (Hagerstown). 2017;18(3):152–8. https://doi.org/10.2459/JCM.0000000000000424.
Flores L, Vidal J, Núñez I, Rueda S, Viaplana J, Esmatjes E. Longitudinal changes of blood pressure after weight loss: factors involved. Surg Obes Relat Dis. 2015;11(1):215–21. https://doi.org/10.1016/j.soard.2014.04.028.
Mertens IL, Van Gaal LF. Overweight, obesity, and blood pressure: the effects of modest weight reduction. Obes Res. 2000;8(3):270–8. https://doi.org/10.1038/oby.2000.32.
Davis BR, Blaufox MD, Oberman A, Wassertheil-Smoller S, Zimbaldi N, Cutler JA, et al. Reduction in long-term antihypertensive medication requirements. Effects of weight reduction by dietary intervention in overweight persons with mild hypertension. Arch Intern Med. 1993;153(15):1773–82. https://doi.org/10.1001/archinte.153.15.1773.
Davy KP, Hall JE. Obesity and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol. 2004;286(5):R803–13. https://doi.org/10.1152/ajpregu.00707.2003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension and Obesity
Rights and permissions
About this article
Cite this article
Samson, R., Ayinapudi, K., Le Jemtel, T.H. et al. Obesity, Hypertension, and Bariatric Surgery. Curr Hypertens Rep 22, 46 (2020). https://doi.org/10.1007/s11906-020-01049-x
Published:
DOI: https://doi.org/10.1007/s11906-020-01049-x