Abstract
Purpose of Review
Tyrosine kinase inhibitors (TKIs) allow many patients with chronic myeloid leukemia (CML) to live normal life spans but have the potential to impact patients’ health-related quality of life (HRQOL). Patient-reported outcome (PRO) measures can provide valuable information to inform treatment decision-making. Here, we review pivotal studies that used PRO measures to evaluate HRQOL of patients with CML in the first-line and treatment-free remission (TFR), and identify areas for future research.
Recent Findings
PRO measures commonly studied in patients with CML include the SF-36, FACT-Leu, EORTC QLQ-CML24, and MDASI CML. Cohort or cross-sectional studies provide the most data on PRO measures in patients with CML, with less information available from randomized controlled trials (RCTs). Patients with CML taking TKIs have worse HRQOL compared to matched controls, with a few studies seeing a larger effect in younger patients (< 60 years old). No single TKI consistently has better HRQOL compared to other agents. Fatigue is a predominant symptom associated with impaired HRQOL across many studies. Studies evaluating TFR show stable or improved HRQOL after TKI discontinuation. There are areas of HRQOL detrimental to patients with other types of cancer (e.g., cognition, sexuality) that warrant further evaluation in patients with CML.
Summary
Understanding the HRQOL of patients with CML is increasingly important as patients live near-normal life expectancies. PRO measures have the potential to inform treatment decisions in this patient population. Future research opportunities include using PRO measures in RCTs and expanding the HRQOL topics studied in patients with CML.
Similar content being viewed by others
References
Papers of particular interest, recently published, are highlighted as: • Of importance
Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385(9976):1447–59. https://doi.org/10.1016/S0140-6736(13)62120-0.
Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM-L. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. JCO. 2016;34(24):2851–7. https://doi.org/10.1200/JCO.2015.66.2866.
Radivoyevitch T, Weaver D, Hobbs B, et al. Do persons with chronic myeloid leukaemia have normal or near normal survival? Leukemia. 2020;34(2):333–5. https://doi.org/10.1038/s41375-019-0699-y.
Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118(12):3123–7. https://doi.org/10.1002/cncr.26679.
US Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. US Department of Health and Human Services Food and Drug Administration. https://www.fda.gov/media/77832/download. Accessed 21 Oct 2020.
Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–9.
Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76.
Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. https://doi.org/10.1016/j.jclinepi.2015.08.038.
Phillips KM, Pinilla-Ibarz J, Sotomayor E, et al. Quality of life outcomes in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors: a controlled comparison. Support Care Cancer. 2013;21(4):1097–103. https://doi.org/10.1007/s00520-012-1630-5.
Efficace F, Baccarani M, Breccia M, et al. Health-related quality of life in chronic myeloid leukemia patients receiving long-term therapy with imatinib compared with the general population. Blood. 2011;118(17):4554–60. https://doi.org/10.1182/blood-2011-04-347575.
Efficace F, Baccarani M, Breccia M, et al. Chronic fatigue is the most important factor limiting health-related quality of life of chronic myeloid leukemia patients treated with imatinib. Leukemia. 2013;27(7):1511–9. https://doi.org/10.1038/leu.2013.51.
Guérin A, Chen L, Ionescu-Ittu R, et al. Impact of low-grade adverse events on health-related quality of life in adult patients receiving imatinib or nilotinib for newly diagnosed Philadelphia chromosome positive chronic myelogenous leukemia in chronic phase. Curr Med Res Opin. 2014;30(11):2317–28. https://doi.org/10.1185/03007995.2014.944973.
Yu L, Wang H, Milijkovic D, Huang X, Jiang Q. Achieving optimal response at 12 months is associated with a better health-related quality of life in patients with chronic myeloid leukemia: a prospective, longitudinal, single center study. BMC Cancer. 2018;18(1):782. https://doi.org/10.1186/s12885-018-4699-5.
Trask PC, Cella D, Besson N, Kelly V, Masszi T, Kim D-W. Health-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemia. Leuk Res. 2012;36(4):438–42. https://doi.org/10.1016/j.leukres.2011.10.011.
Trask PC, Cella D, Powell C, Reisman A, Whiteley J, Kelly V. Health-related quality of life in chronic myeloid leukemia. Leuk Res. 2013;37(1):9–13. https://doi.org/10.1016/j.leukres.2012.09.013.
Whiteley J, Reisman A, Shapiro M, Cortes JE, Cella D. Health-related quality of life during bosutinib (SKI-606) therapy in patients with advanced chronic myeloid leukemia after imatinib failure. Curr Med Res Opin. 2016;32(8):1325–34. https://doi.org/10.1185/03007995.2016.1174108.
• Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Patient-reported outcomes in the phase 3 BFORE trial of bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia. J Cancer Res Clin Oncol. 2019;145(6):1589–99. https://doi.org/10.1007/s00432-019-02894-3. The phase III BFORE trial is the largest RCT in CML to utilize PRO measures. The BFORE trial evaluated bosutinib vs imatinib in nearly 500 patients with new diagnoses of CML using the FACT-Leu and EQ-5D. There were no significant differences between patients taking imatinib or bosutinib in the first-line setting, and findings supported the maintenance or improvement in HRQOL by 12 months.
Brümmendorf TH, Gambacorti-Passerini C, Bushmakin AG, et al. Relationship between molecular response and quality of life with bosutinib or imatinib for chronic myeloid leukemia. Ann Hematol. 2020;99(6):1241–9. https://doi.org/10.1007/s00277-020-04018-1.
Efficace F, Baccarani M, Breccia M, et al. International development of an EORTC questionnaire for assessing health-related quality of life in chronic myeloid leukemia patients: the EORTC QLQ-CML24. Qual Life Res. 2014;23(3):825–36. https://doi.org/10.1007/s11136-013-0523-5.
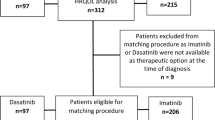
Efficace F, Stagno F, Iurlo A, et al. Health-related quality of life of newly diagnosed chronic myeloid leukemia patients treated with first-line dasatinib versus imatinib therapy. Leukemia. 2020;34(2):488–98. https://doi.org/10.1038/s41375-019-0563-0.
Tan BK, Chua SS, Chen L-C, Chang KM, Balashanker S, Bee PC. Efficacy of a medication management service in improving adherence to tyrosine kinase inhibitors and clinical outcomes of patients with chronic myeloid leukaemia: a randomised controlled trial. Support Care Cancer. 2020;28(7):3237–47. https://doi.org/10.1007/s00520-019-05133-0.
Williams LA, Garcia Gonzalez AG, Ault P, et al. Measuring the symptom burden associated with the treatment of chronic myeloid leukemia. Blood. 2013;122(5):641–7. https://doi.org/10.1182/blood-2013-01-477687.
Cortes JE, Lipton JH, Miller CB, et al. Evaluating the impact of a switch to nilotinib on imatinib-related chronic low-grade adverse events in patients with CML-CP: the ENRICH study. Clin Lymphoma Myeloma Leuk. 2016;16(5):286–96. https://doi.org/10.1016/j.clml.2016.02.002.
• Zulbaran-Rojas A, Lin HK, Shi Q, et al. A prospective analysis of symptom burden for patients with chronic myeloid leukemia in chronic phase treated with frontline second- and third-generation tyrosine kinase inhibitors. Cancer Med. 2018;7(11):5457–69. https://doi.org/10.1002/cam4.1808. This paper provides the only available PRO measure information on patients taking ponatinib in the first-line setting.
Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–9. https://doi.org/10.1056/NEJMoa0912614.
Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus Imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362(24):2260–70. https://doi.org/10.1056/NEJMoa1002315.
Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus Imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE Trial. JCO. 2017;36(3):231–7. https://doi.org/10.1200/JCO.2017.74.7162.
Labeit AM, Copland M, Cork LM, et al. Assessment of quality of life in the NCRI Spirit 2 study comparing imatinib with dasatinib in patients with newly-diagnosed chronic phase chronic myeloid leukaemia. Blood. 2015;126(23):4024–4024. https://doi.org/10.1182/blood.V126.23.4024.4024.
Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30(28):3486–92. https://doi.org/10.1200/JCO.2011.38.7522.
Lipton JH, Trask PC, Cella D, et al. Health-related quality of life (HRQoL) in newly diagnosed patients (pts) with chronic phase chronic myelogenous leukemia (CP CML) treated with bosutinib (BOS) or imatinib (IM). JCO. 2011;29(15_suppl):6612–6612. https://doi.org/10.1200/jco.2011.29.15_suppl.6612.
Jain P, Kantarjian H, Jabbour E, et al. Ponatinib as first-line treatment for patients with chronic myeloid leukaemia in chronic phase: a phase 2 study. Lancet Haematol. 2015;2(9):e376–83.
Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404.
Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–7.
Etienne G, Guilhot J, Rea D, et al. Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. JCO. 2016;35(3):298–305. https://doi.org/10.1200/JCO.2016.68.2914.
Ross DM, Pagani IS, Shanmuganathan N, et al. Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32(12):2572–9. https://doi.org/10.1038/s41375-018-0264-0.
Rea D, Nicolini FE, Tulliez M, et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129(7):846–54. https://doi.org/10.1182/blood-2016-09-742205.
Ross DM, Masszi T, Gómez Casares MT, et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144(5):945–54. https://doi.org/10.1007/s00432-018-2604-x.
Saussele S, Richter J, Guilhot J, et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19(6):747–57. https://doi.org/10.1016/S1470-2045(18)30192-X.
Shah NP, García-Gutiérrez V, Jiménez-Velasco A, et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: the DASFREE study. Leuk Lymphoma. 2019:1–10. https://doi.org/10.1080/10428194.2019.1675879
Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. JCO. 2013;32(5):424–30. https://doi.org/10.1200/JCO.2012.48.5797.
National Comprehensive Cancer Network. Chronic Myeloid Leukemia (Version 3.2020). https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed 22 Oct 2020.
Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–84. https://doi.org/10.1038/s41375-020-0776-2.
Park JS, Lee S-E, Jeong SH, et al. Change of health-related profiles after Imatinib cessation in chronic phase chronic myeloid leukemia patients. Leuk Lymphoma. 2016;57(2):341–7. https://doi.org/10.3109/10428194.2015.1049166.
Mori S, Vagge E, le Coutre P, et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am J Hematol. 2015;90(10):910–4. https://doi.org/10.1002/ajh.24120.
Mori S, le Coutre P, Abruzzese E, et al. Imatinib Suspension and Validation (ISAV) study: final results at 79 months. Blood. 2018;132(Supplement 1):461–461. https://doi.org/10.1182/blood-2018-99-112982.
Hochhaus A, Masszi T, Giles FJ, et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: results from the ENESTfreedom study. Leukemia. 2017;31(7):1525–31. https://doi.org/10.1038/leu.2017.63.
Clark RE, Polydoros F, Apperley JF, et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017;4(7):e310–6. https://doi.org/10.1016/S2352-3026(17)30066-2.
Clark RE, Polydoros F, Apperley JF, et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 2019;6(7):e375–83. https://doi.org/10.1016/S2352-3026(19)30094-8.
• Atallah E, Schiffer CA, Radich JP, et al. Assessment of outcomes after stopping tyrosine kinase inhibitors among patients with chronic myeloid leukemia: a nonrandomized clinical trial. JAMA Oncol. 2021;7(1):42–50. https://doi.org/10.1001/jamaoncol.2020.5774. This is the only paper to date to use PROMIS measures in patients with CML and is the largest study in the U.S. to evaluate HRQOL in patients attempting TFR.
Hardy SJ, Krull KR, Wefel JS, Janelsins M. Cognitive changes in cancer survivors. Am Soc Clin Oncol Educ Book. 2018;38:795–806. https://doi.org/10.1200/EDBK_201179.
Efficace F, Breccia M, Saussele S, et al. Which health-related quality of life aspects are important to patients with chronic myeloid leukemia receiving targeted therapies and to health care professionals? GIMEMA and EORTC Quality of Life Group. Ann Hematol. 2012;91(9):1371–81.
Pemmaraju N, Kantarjian HM, Tanaka MF, et al. Memory impairment in chronic phase (CP) chronic myeloid leukemia (CML) patients (pts) treated with dasatinib tyrosine kinase inhibitor (TKI) therapy. Blood. 2011;118(21):3771–3771. https://doi.org/10.1182/blood.V118.21.3771.3771.
Schoenbeck KL, Tummala S, Goyal NG, Smith CC, Shah NP. Cognitive dysfunction associated with tyrosine kinase inhibitors in patients with CML in chronic phase. Blood. 2020;136(Supplement 1):2–3.
Meadows ME, Chang G, Jones JA, Antin JR, Orav EJ. Predictors of neuropsychological change in patients with chronic myelogenous leukemia and myelodysplastic syndrome. Arch Clin Neuropsychol. 2013;28(4):363–74. https://doi.org/10.1093/arclin/acs141.
Schover LR. Sexuality and fertility after cancer. Hematology. 2005;2005(1):523–7. https://doi.org/10.1182/asheducation-2005.1.523.
Niscola P, Efficace F, Abruzzese E. Sexual health in patients with hematological malignancies: a neglected issue. Support Care Cancer. 2018;26(6):1699–701. https://doi.org/10.1007/s00520-018-4124-2.
ShachamAbulafia A, Shemesh S, Rosenmann L, et al. Health-related quality of life in patients with chronic myeloid leukemia treated with first- versus second-generation tyrosine kinase inhibitors. J Clin Med. 2020;9(11):3417. https://doi.org/10.3390/jcm9113417. Published 2020 Oct 25.
Novartis. Gleevac (imatinib) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021588s024lbl.pdf. Accessed 10 Dec 2020.
Poorvu PD, Frazier AL, Feraco AM, et al. Cancer treatment-related infertility: a critical review of the evidence. JNCI Cancer Spectrum. 2019;3(pkz008). https://doi.org/10.1093/jncics/pkz008
Abruzzese E, Trawinska MM, de Fabritiis P, Baccarani M. Management of pregnant chronic myeloid leukemia patients. Expert Rev Hematol. 2016;9(8):781–91. https://doi.org/10.1080/17474086.2016.1205479.
Abruzzese E, Turkina AG, Apperley JF, et al. Pregnancy management in CML patients: to treat or not to treat? Report of 224 Outcomes of the European Leukemia Net (ELN) Database. Blood. 2019;134(Supplement_1):498–498. https://doi.org/10.1182/blood-2019-124430.
Zafar SY, Abernethy AP. Financial toxicity, Part I: a new name for a growing problem. Oncology (Williston Park). 2013;27(2):80–149.
Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood. 2013;121(22):4439–42.
Kenzik KM, Bhatia R, Bhatia S. Expenditures for first- and second-generation tyrosine kinase inhibitors before and after transition of imatinib to generic status. JAMA Oncol. 2020;6(4):542–6.
Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–11.
Winn AN, Keating NL, Dusetzina SB. Factors associated with tyrosine kinase inhibitor initiation and adherence among medicare beneficiaries with chronic myeloid leukemia. J Clin Oncol. 2016;34(36):4323–8.
Seymour EK, Ruterbusch JJ, Winn AN, George JA, Beebe-Dimmer JL, Schiffer CA. The costs of treating and not treating patients with chronic myeloid leukemia with tyrosine kinase inhibitors among Medicare patients in the United States. Cancer. 2021;127(1):93–102.
Kota V, Atallah E. Musculoskeletal pain in patients with chronic myeloid leukemia after tyrosine kinase inhibitor therapy cessation. Clin Lymphoma Myeloma Leuk. 2019;19(8):480–7.
Sogawa R, Kimura S, Yakabe R, et al. Anxiety and depression associated with tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. Int J Clin Oncol. 2018;23(5):974–9. https://doi.org/10.1007/s10147-018-1275-6.
Sharf G, Marin C, Bradley JA, et al. Treatment-free remission in chronic myeloid leukemia: the patient perspective and areas of unmet needs. Leukemia. 2020;34(8):2102–12. https://doi.org/10.1038/s41375-020-0867-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
KE Flynn receives grants from the NIH and Novartis, and performs consulting for ReFocus and Inhibikase. KL Schoenbeck receives research funding from the American Society of Hematology.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Chronic Myeloid Leukemias
Rights and permissions
About this article
Cite this article
Schoenbeck, K.L., Flynn, K.E. Health-Related Quality of Life of Patients with Chronic Myeloid Leukemia as Measured by Patient-Reported Outcomes: Current State and Future Directions. Curr Hematol Malig Rep 16, 491–499 (2021). https://doi.org/10.1007/s11899-021-00656-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00656-y