Abstract
Purpose of the Review
The purpose of this review is to describe the effects of angiotensin receptor neprilysin inhibitor (ARNI) therapy on the natriuretic peptide axis (NPA), with a particular focus on B-type natriuretic peptide (BNP), atrial natriuretic peptide (ANP), and C-type natriuretic peptide (CNP) to better understand the biology behind the improved outcomes in patients with heart failure with reduced ejection fraction (HFrEF).
Recent Findings
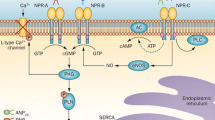
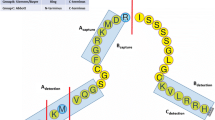
BNP, ANP, and CNP are the three main natriuretic peptides (NP); they share a common structure and ultimately mediate their actions by activating cyclic guanosine monophosphate (cGMP). ARNI therapy results in a decrease of N-terminal pro-BNP (NT-proBNP) and increase of BNP levels respectively. It is been questioned whether these changes may result from unique laboratory assays characteristics rather than actual biological implications. It appears to be that the prognostic accuracy of BNP for cardiovascular outcomes remains independent and comparable to that of NT-proBNP while on ARNI therapy. ANP levels also increase with ARNI therapy, but no consistent change has been described for CNP levels. There is evidence that the changes in BNP and NT-proBNP correlate with improvement in echocardiographic parameters of volume and function.
Summary
The dual effect of neprilysin inhibition and angiotensin receptor blockade has substantial implications on the natriuretic peptide axis (NPA). The changes seen in BNP and NT-proBNP specifically have shown to correlate with improvement in echocardiographic parameters. Further results exploring the biologic effects of ARNI therapy on other NPs are still pending and likely will provide further insights in the mechanisms behind the improvement in cardiac function and clinical outcomes.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–239.
Writing Committee M, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2016;134(13):e282–93.
Yancy CW, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017;136(6):e137–61.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50(25):2357–68.
Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68(6):639–53.
Ibrahim NE, Januzzi JL Jr. Beyond natriuretic peptides for diagnosis and Management of Heart Failure. Clin Chem. 2017;63(1):211–22.
Knecht M, Pagel I, Langenickel T, Philipp S, Scheuermann-Freestone M, Willnow T, et al. Increased expression of renal neutral endopeptidase in severe heart failure. Life Sci. 2002;71(23):2701–12.
McMurray JJ, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Mangiafico S, et al. Neutral endopeptidase inhibition and the natriuretic peptide system: an evolving strategy in cardiovascular therapeutics. Eur Heart J. 2013;34(12):886–893c.
Gu J, Noe A, Chandra P, al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–14.
Menendez JT. The mechanism of action of LCZ696. Card Fail Rev. 2016;2(1):40–6.
Jhund PS, McMurray JJ. The neprilysin pathway in heart failure: a review and guide on the use of sacubitril/valsartan. Heart. 2016;102(17):1342–7.
Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195–203.
de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28(1):89–94.
Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332(6159):78–81.
Yoshimura M, Yasue H, Okumura K, Ogawa H, Jougasaki M, Mukoyama M, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87(2):464–9.
Ackermann DM, et al. Atrial natriuretic peptide: localization in the human heart. JAMA. 1986;256(8):1048.
Tonne JM, Campbell JM, Cataliotti A, Ohmine S, Thatava T, Sakuma T, et al. Secretion of glycosylated pro-B-type natriuretic peptide from normal cardiomyocytes. Clin Chem. 2011;57(6):864–73.
Silver MA, Maisel A, Yancy CW, McCullough PA, Burnett JC, Francis GS, et al. BNP consensus panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases. Congest Heart Fail. 2004;10(5 Suppl 3):1–30.
Heublein DM, Clavell AL, Stingo AJ, Lerman A, Wold L, Burnett JC Jr. C-type natriuretic peptide immunoreactivity in human breast vascular endothelial cells. Peptides. 1992;13(5):1017–9.
Cataliotti A, Giordano M, de Pascale E, Giordano G, Castellino P, Jougasaki M, et al. CNP production in the kidney and effects of protein intake restriction in nephrotic syndrome. Am J Physiol Renal Physiol. 2002;283(3):F464–72.
Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87(4):1402–12.
Chen HH, Cataliotti A, Schirger JA, Martin FL, Burnett JC Jr. Equimolar doses of atrial and brain natriuretic peptides and urodilatin have differential renal actions in overt experimental heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1093–7.
Chauhan SD, Hobbs AJ, Ahluwalia A. C-type natriuretic peptide: new candidate for endothelium-derived hyperpolarising factor. Int J Biochem Cell Biol. 2004;36(10):1878–81.
Moyes AJ, Hobbs AJ. C-type Natriuretic Peptide: A Multifaceted Paracrine Regulator in the Heart and Vasculature. Int J Mol Sci. 2019;20(9):2281
Ibrahim N, Januzzi JL. The potential role of natriuretic peptides and other biomarkers in heart failure diagnosis, prognosis and management. Expert Rev Cardiovasc Ther. 2015;13(9):1017–30.
Nakao K, et al. Molecular biology and biochemistry of the natriuretic peptide system. I: natriuretic peptides. J Hypertens. 1992;10(9):907–12.
Kuhn M. Molecular physiology of natriuretic peptide signalling. Basic Res Cardiol. 2004;99(2):76–82.
Cataliotti A, Schirger JA, Martin FL, Chen HH, McKie PM, Boerrigter G, et al. Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation. 2005;112(6):836–40.
Potter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278(11):1808–17.
Smith MW, Espiner EA, Yandle TG, Charles CJ, Richards AM. Delayed metabolism of human brain natriuretic peptide reflects resistance to neutral endopeptidase. J Endocrinol. 2000;167(2):239–46.
Charles CJ, et al. Clearance receptors and endopeptidase 24.11: equal role in natriuretic peptide metabolism in conscious sheep. Am J Phys. 1996;271(2 Pt 2):R373–80.
Ng LL, et al. Diagnosis of heart failure using urinary natriuretic peptides. Clin Sci (Lond). 2004;106(2):129–33.
Schou M, Gustafsson F, Kistorp CN, Corell P, Kjaer A, Hildebrandt PR. Effects of body mass index and age on N-terminal pro brain natriuretic peptide are associated with glomerular filtration rate in chronic heart failure patients. Clin Chem. 2007;53(11):1928–35.
Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, et al. B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis. 2005;46(4):610–20.
Maisel AS, Daniels LB, Anand IS, McCullough PA, Chow SL. Utility of natriuretic peptides to assess and manage patients with heart failure receiving angiotensin receptor blocker/neprilysin inhibitor therapy. Postgrad Med. 2018;130(3):299–307.
Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both. J Am Coll Cardiol. 2007;49(10):1089–91.
Hawkridge AM, Heublein DM, Bergen HR, Cataliotti A, Burnett JC, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102(48):17442–7.
Niederkofler EE, Kiernan UA, O'Rear J, Menon S, Saghir S, Protter AA, et al. Detection of endogenous B-type natriuretic peptide at very low concentrations in patients with heart failure. Circ Heart Fail. 2008;1(4):258–64.
Miller WL, Phelps MA, Wood CM, Schellenberger U, van le A, Perichon R, et al. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4(3):355–60.
Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, et al. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316(1–2):129–35.
Liang F, O’Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, et al. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49(10):1071–8.
Diez J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: implications for therapy. Eur J Heart Fail. 2017;19(2):167–76.
Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49(10):1079–88.
Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–8.
Fielitz J, Dendorfer A, Pregla R, Ehler E, Zurbrügg HR, Bartunek J, et al. Neutral endopeptidase is activated in cardiomyocytes in human aortic valve stenosis and heart failure. Circulation. 2002;105(3):286–9.
McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8(1):79–84.
Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351(9116):1657–8.
Burnett JC Jr. Vasopeptidase inhibition: a new concept in blood pressure management. J Hypertens Suppl. 1999;17(1):S37–43.
Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–95.
•• Packer M, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61 Large scale study from surviving patients enrolled in the PARADIGM-HF trial comparing BNP, NT-proBNP, and urinary cGMP levels in the ARNI versus enalapril treatment group.
Ibrahim NE, McCarthy CP, Shrestha S, Gaggin HK, Mukai R, Szymonifka J, et al. Effect of neprilysin inhibition on various natriuretic peptide assays. J Am Coll Cardiol. 2019;73(11):1273–84.
Nougue H, et al. Effects of sacubitril/valsartan on neprilysin targets and the metabolism of natriuretic peptides in chronic heart failure: a mechanistic clinical study. Eur J Heart Fail. 2019;21(5):598–605.
Velazquez EJ, Morrow DA, DeVore A, Duffy CI, Ambrosy AP, McCague K, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539–48.
•• Januzzi JL, Jr., Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, et al. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322(11):1085-1095. Exploratory study of HFrEF patients treated with ARNI therapy showing a correlation between NT-proBNP reduction and reverse cardiac remodeling.
• Myhre PL, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73(11):1264–72 Study investigating the prognostic performance of BNP measurements compared to NT-proBNP in a subset of HFrEF patients from the PARADIGM-HF trial started on ARNI therapy.
Ibrahim NE, Januzzi JL. Monitoring biomarkers in patients receiving Neprilysin inhibitors. Current Emergency and Hospital Medicine Reports. 2018;6(1):8–16.
Norman JA, Little D, Bolgar M, di Donato G. Degradation of brain natriuretic peptide by neutral endopeptidase: species specific sites of proteolysis determined by mass spectrometry. Biochem Biophys Res Commun. 1991;175(1):22–30.
Jaffe AS, Apple FS, Mebazaa A, Vodovar N. Unraveling N-terminal pro-B-type natriuretic peptide: another piece to a very complex puzzle in heart failure patients. Clin Chem. 2015;61(8):1016–8.
Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of markers of cardiac damage. Clin Chem. 2008;54(3):619–21.
Kobalava Z, Kotovskaya Y, Averkov O, Pavlikova E, Moiseev V, Albrecht D, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther. 2016;34(4):191–8.
Pankow K, Schwiebs A, Becker M, Siems WE, Krause G, Walther T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic peptide degradation. J Mol Biol. 2009;393(2):496–503.
D’Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19(6):710–7.
Yancy CW, et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018;71(2):201–30.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Biomarkers of Heart Failure
Rights and permissions
About this article
Cite this article
Vasquez, N., Carter, S. & Grodin, J.L. Angiotensin Receptor–Neprilysin Inhibitors and the Natriuretic Peptide Axis. Curr Heart Fail Rep 17, 67–76 (2020). https://doi.org/10.1007/s11897-020-00458-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11897-020-00458-y