Abstract
Purpose of Review
There is consensus that metformin should be the first-line pharmacological therapy for type 2 diabetes. Although new evidence on effective treatments for type 2 diabetes is rapidly evolving, there is uncertainty regarding the optimal choice of second-line therapy. Our aim was to review the current major guidelines for second-line therapy in type 2 diabetes, along with findings from the recent cardiovascular outcome trials, focusing on two particularly promising classes of glucose-lowering drugs, sodium-glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 receptor agonists (GLP1 RAs).
Recent Findings
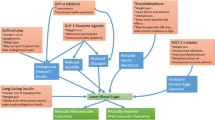
In the recent randomized controlled trials, two SGLT2 inhibitors (i.e., empagliflozin and canagliflozin) and two GLP1 RAs (i.e., liraglutide and albiglutide) reduced cardiovascular events in patients with type 2 diabetes, of whom most had established atherosclerotic cardiovascular disease. Some clinical guidelines have changed their recommendations for second-line therapy based on these findings. The first choice for a second-line therapy by the new American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) guidelines is SGLT2 inhibitors or GLP1 RAs for patients with atherosclerotic cardiovascular disease, heart failure, or chronic kidney disease. For patients without these conditions, the ADA/EASD lists five options of noninsulin second-line therapy without a suggested hierarchy of use. On the other hand, the 2019 consensus statement from the American Association of Clinical Endocrinologists/American College of Endocrinology lists nine hierarchical options, with GLP1 RAs as the first recommended therapy, followed by SGLT2 inhibitors and dipeptidyl peptidase 4 (DPP4) inhibitors, and sulfonylurea as the last option. The American College of Physicians recommends four oral treatment options, which do not include GLP1 RAs. The International Diabetes Federation recommends sulfonylureas, DPP4 inhibitors, or SGLT2 inhibitors as preferred second-line drugs with GLP1 RAs as an alternative in obese patients. The World Health Organization strongly recommends sulfonylureas in low-resource settings. The National Institute for Health and Care Excellence in the UK recommends DPP4 inhibitors, thiazolidinediones, or sulfonylureas, with use of SGLT2 inhibitors only under special circumstances.
Summary
Clinical guidelines for the choice of second-line therapy in type 2 diabetes are inconsistent. A comprehensive assessment of the risks and benefits of second-line therapy is needed to address knowledge gaps that underlie core clinical practice.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. https://doi.org/10.1016/j.diabres.2018.02.023.
Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017.
Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–44. https://doi.org/10.2337/dc12-0002.
Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–50. https://doi.org/10.1001/jama.291.7.844.
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. https://doi.org/10.1161/01.cir.0000437741.48606.98.
Shen Y, Cai R, Sun J, Dong X, Huang R, Tian S, et al. Diabetes mellitus as a risk factor for incident chronic kidney disease and end-stage renal disease in women compared with men: a systematic review and meta-analysis. Endocrine. 2017;55(1):66–76. https://doi.org/10.1007/s12020-016-1014-6.
American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–28. https://doi.org/10.2337/dci18-0007.
American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S90–S102. https://doi.org/10.2337/dc19-S009.
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2019 executive summary. Endocr Pract. 2019;25(1):69–100. https://doi.org/10.4158/CS-2018-0535.
Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701. https://doi.org/10.2337/dci18-0033.
Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Clinical Guidelines Committee of the American College of P. oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166(4):279–90. https://doi.org/10.7326/M16-1860.
International Diabetes Federation. Recommendations for managing type 2 diabetes in primary care, 2017. www.idf.org/managing-type2-diabetes. Accessed 14 Apr 2019.
Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–25. https://doi.org/10.1007/s00125-016-4157-3.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28. https://doi.org/10.1056/NEJMoa1504720.
•• Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. https://doi.org/10.1056/NEJMc1712572. Findings from the CANVAS program suggest that canagliflozin reduces a risk of cardiovascular events, but increases a risk of amputation in patients with type 2 diabetes and an elevated risk of cardiovascular disease.
Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–57. https://doi.org/10.1056/NEJMoa1812389.
Das SR, Everett BM, Birtcher KK, Brown JM, Cefalu WT, Januzzi JL Jr, et al. 2018 ACC expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes and atherosclerotic cardiovascular disease: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2018;72(24):3200–23. https://doi.org/10.1016/j.jacc.2018.09.020.
Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–9. https://doi.org/10.1016/S0140-6736(18)32590-X.
National Institutes of Health. Study to evaluate the effect of dapagliflozin on the incidence of worsening heart failure or cardiovascular death in patients with chronic heart failure (Dapa-HF). Identifier: NCT03036124. https://www.clinicaltrials.gov. Accessed 15 Apr 2019.
National Institutes of Health. EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with preserved ejection fraction (EMPEROR-Preserved). Identifier: NCT03057951. https://www.clinicaltrials.gov. Accessed 15 April 2019.
National Institutes of Health. EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure with reduced ejection fraction (EMPEROR-reduced). Identifier: NCT03057977. https://www.clinicaltrials.gov. Accessed 15 Apr 2019.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–57. https://doi.org/10.1056/NEJMoa1611925.
Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–34. https://doi.org/10.1056/NEJMoa1515920.
Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46(6):462–72. https://doi.org/10.1159/000484633.
•• Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019. https://doi.org/10.1056/NEJMoa1811744. Findings from the CREDENCE trial suggest that canagliflozin reduces the risk of kidney failure and cardiovascular events in patients with type 2 diabetes and kidney disease.
Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, et al. The potential for improving cardio-renal outcomes by sodium-glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J. 2018;11(6):749–61. https://doi.org/10.1093/ckj/sfy090.
National Institutes of Health. A study to evaluate the effect of dapagliflozin on renal outcomes and cardiovascular mortality in patients with chronic kidney disease (Dapa-CKD). Identifier: NCT03036150. https://www.clinicaltrials.gov. Accessed 15 Apr 2019.
Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab. 2015;6(1):19–28. https://doi.org/10.1177/2042018814559725.
•• Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. https://doi.org/10.1056/NEJMoa1603827. Findings from the LEADER trial suggest that liraglutide reduces a risk of cardiovascular events in patients with type 2 diabetes; most of whom had established atherosclerotic cardiovascular disease.
Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–44. https://doi.org/10.1056/NEJMoa1607141.
Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228–39. https://doi.org/10.1056/NEJMoa1612917.
Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–57. https://doi.org/10.1056/NEJMoa1509225.
Hernandez AF, Green JB, Janmohamed S, D’Agostino RB Sr, Granger CB, Jones NP, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392(10157):1519–29. https://doi.org/10.1016/S0140-6736(18)32261-X.
Bain SC, Mosenzon O, Arechavaleta R, Bogdanski P, Comlekci A, Consoli A, et al. Cardiovascular safety of oral semaglutide in patients with type 2 diabetes: rationale, design and patient baseline characteristics for the PIONEER 6 trial. Diabetes Obes Metab. 2019;21(3):499–508. https://doi.org/10.1111/dom.13553.
Company announcement: oral semaglutide demonstrates a favourable cardiovascular safety profile and a significant reduction in cardiovascular death and all-cause mortality in people with type 2 diabetes in the PIONEER 6 trial. https://www.novonordisk.com/bin/getPDF.2226789.pdf. Accessed 14 Apr 2019.
Jorsal A, Kistorp C, Holmager P, Tougaard RS, Nielsen R, Hanselmann A, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19(1):69–77. https://doi.org/10.1002/ejhf.657.
Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316(5):500–8. https://doi.org/10.1001/jama.2016.10260.
Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Design and baseline characteristics of participants in the Researching cardiovascular Events with a Weekly INcretin in Diabetes (REWIND) trial on the cardiovascular effects of dulaglutide. Diabetes Obes Metab. 2018;20(1):42–9. https://doi.org/10.1111/dom.13028.
Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(9):839–48. https://doi.org/10.1056/NEJMoa1616011.
Bethel MA, Mentz RJ, Merrill P, BUSE JB, Chan JC, Goodman SG, et al. Renal outcomes in the EXenatide Study of Cardiovascular Event Lowering (EXSCEL). Diabetes Care. 2018;67(Supplement 1):522–P. https://doi.org/10.2337/db18-522-P.
Muskiet MHA, Tonneijck L, Huang Y, Liu M, Saremi A, Heerspink HJL, et al. Lixisenatide and renal outcomes in patients with type 2 diabetes and acute coronary syndrome: an exploratory analysis of the ELIXA randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):859–69. https://doi.org/10.1016/S2213-8587(18)30268-7.
Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373(9681):2125–35. https://doi.org/10.1016/S0140-6736(09)60953-3.
Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279–89. https://doi.org/10.1016/S0140-6736(05)67528-9.
Gaziano JM, Cincotta AH, Vinik A, Blonde L, Bohannon N, Scranton R. Effect of bromocriptine-QR (a quick-release formulation of bromocriptine mesylate) on major adverse cardiovascular events in type 2 diabetes subjects. J Am Heart Assoc. 2012;1(5):e002279. https://doi.org/10.1161/JAHA.112.002279.
Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–42. https://doi.org/10.1056/NEJMoa1501352.
Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–26. https://doi.org/10.1056/NEJMoa1307684.
White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327–35. https://doi.org/10.1056/NEJMoa1305889.
Rosenstock J, Perkovic V, Johansen OE, Cooper ME, Kahn SE, Marx N, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69–79. https://doi.org/10.1001/jama.2018.18269.
FDA. Endocrinologic and Metabolic Drug Advisory Committee. 2008; Day 2, Part 1 (1–100).
FDA. Endocrinologic and Metabolic Drug Advisory Committee. 2008; Day 2, Part 2 (101–200).
FDA. Endocrinologic and Metabolic Drug Advisory Committee. 2008; Day 2, Part 3 (201–300).
Taylor SI, Leslie BR. Cardiovascular outcome trials of diabetes drugs: lessons learned. J Clin Invest. 2018;128(3):893–6. https://doi.org/10.1172/JCI99820.
Nathan DM, Buse JB, Kahn SE, Krause-Steinrauf H, Larkin ME, Staten M, et al. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254–61. https://doi.org/10.2337/dc13-0356.
World Health Organization. Guidelines on second- and third-line medicines and type of insulin for the control of blood glucose levels in non-pregnant adults with diabetes mellitus. 2018. https://www.who.int/diabetes/publications/guidelines-diabetes-medicines/en/. Accessed 15 Apr 2019.
Peyrot M, Rubin RR, Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Prim Care Diabetes. 2010;4(Suppl 1):S11–8. https://doi.org/10.1016/S1751-9918(10)60004-6.
Ishii H, Iwamoto Y, Tajima N. An exploration of barriers to insulin initiation for physicians in Japan: findings from the Diabetes Attitudes, Wishes and Needs (DAWN) JAPAN study. PLoS One. 2012;7(6):e36361. https://doi.org/10.1371/journal.pone.0036361.
National Institute for Health and Care Excellence. Managing blood glucose in adults with type 2 diabetes: NICE Pathways. 2019. https://pathways.nice.org.uk/pathways/type-2-diabetes-in-adults#path=view%3A/pathways/type-2-diabetes-in-adults/managing-blood-glucose-in-adults-with-type-2-diabetes.xml&content=view-index. Accessed 15 Apr 2019.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management: evidence reviews for SGLT-2 inhibitors and GLP-1 mimetics. 2018. https://www.nice.org.uk/guidance/ng28/evidence/march-2018-evidence-reviews-for-sglt2-inhibitors-and-glp1-mimetics-pdf-4783687597. Accessed 15 Apr 2019.
Funding
This project is funded by NIH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The author declares that she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Diabetes Epidemiology
Rights and permissions
About this article
Cite this article
Shin, JI. Second-line Glucose-Lowering Therapy in Type 2 Diabetes Mellitus. Curr Diab Rep 19, 54 (2019). https://doi.org/10.1007/s11892-019-1171-0
Published:
DOI: https://doi.org/10.1007/s11892-019-1171-0