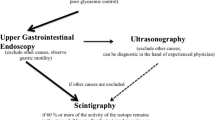
Abstract
In contrast to gastric dysfunction, diabetes-related functional impairments of the small and large intestine have been studied less intensively. The gastrointestinal tract accomplishes several functions, such as mixing and propulsion of luminal content, absorption and secretion of ions, water, and nutrients, defense against pathogens, and elimination of waste products. Diverse functions of the gut are regulated by complex interactions among its functional elements, including gut microbiota. The network-forming tissues, the enteric nervous system) and the interstitial cells of Cajal, are definitely impaired in diabetic patients, and their loss of function is closely related to the symptoms in diabetes, but changes of other elements could also play a role in the development of diabetes mellitus-related motility disorders. The development of our understanding over the recent years of the diabetes-induced dysfunctions in the small and large intestine are reviewed in this article.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–84.
Schvarcz E, Palmér M, Ingberg CM, et al. Increased prevalence of upper gastrointestinal symptoms in long-term type 1 diabetes mellitus. Diabet Med. 1996;13:478–81.
von der Ohe MR. Diarrhea in patients with diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:730–6.
Janatuinen E, Pikkarainen P, Laakso M, et al. Gastrointestinal symptoms in middleaged diabetic patients. Scand J Gastroenterol. 1993;28:427–32.
Bytzer P, Talley NJ, Hammer J, et al. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–11.
Talley NJ, Phillips SF, Melton LJI, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4.
Maxton DG, Whorwell PJ. Functional bowel symptoms in diabetes—the role of autonomic neuropathy. Postgrad Med J. 1991;67:991–3.
Wald A. Incontinence and anorectal dysfunction in patients with diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:737–9.
Quan C, Talley NJ, Cross S, et al. Development and validation of the diabetes bowel symptom questionnaire. Aliment Pharmacol Ther. 2003;17:1179–87.
Lelic D, Brock C, Simrém M, et al. The brain networks encoding visceral sensation in patients with gastrointestinal symptoms due to diabetic neuropathy. Neurogastroenterol Motil. 2014;26:46–58.
Lelic D, Brock C, Søfteland E, et al. Brain networks encoding rectal sensation in type 1 diabetes. Neuroscience. 2013;1:96–105.
Brock C, Søfteland E, Gunterberg V, et al. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care. 2013;36:3698–705.
Blair PJ, Rhee P-L, Sanders KM, et al. The significance of interstitial cells in neurogastroenterology. J Neurogastroenterol Motil. 2014;20:294–317. This is an excellent report about the contribution of ICC to the gastrointestinal motility.
Ördög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8–18.
Huizinga JD, Chen J-H, Zhu YF. The origin of segmentation motor activity in the intestine. Nat Commun. 2014;5:1–11.
Huizinga JD, Martz S, Gil V, et al. Two independent networks of interstitial cells of Cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front Neurosci. 2011;5:1–14.
Sjölund K, Sandén G, Håkanson R, et al. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology. 1983;85:1120–30.
Greenberg GR, Pokol-Daniel S. Neural modulation of glucose-dependent insulinotropic peptide (GIP) and insulin secretion in conscious dogs. Pancreas. 1994;9:531–5.
Pappachan JM, Raveendran AV, Sriraman R. Incretin manipulation in diabetes management. World J Diabetes. 2015;6:774–81.
Holst JJ, Burcelin R, Nathanson E. Neuroprotective properties of GLP-1: theoretical and practical applications. Curr Med Res Opin. 2011;27:547–58.
Kazakos KA, Sarafidis PA, Yovos JG. The impact of diabetic autonomic neuropathy on the incretin effect. Med Sci Monit. 2008;14:CR213–20.
Forrest A, Huizinga JD, Wang XY, et al. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315–26.
Imaeda K, Takano H, Koshita M, et al. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1–11.
Huizinga JD, Zarate N, Farrugia G. Physiology, injury and recovery of interstitial cells of Cajal: basic and clinical science. Gastroenterology. 2009;137:1548–56.
Sanders KM, Ördög T, Koh SD, et al. Physiology and pathophysiology of the interstitial cells of Cajal: from bench to bedside. IV. Genetic and animal models of GI motility disorders caused by loss of interstitial cells of Cajal. Am J Physiol Gastrointest Liver Physiol. 2002;282:G747–56.
Yamamoto T, Watabe K, Nakahara M, et al. Involvement of interstitial cells of Cajal in gastrointestinal dysmotility of diabetic db/db mice. Gastroenterology. 2006;130:A-90.
Wim JEP, Lammers HM, Al-Bloushi SA, et al. Slow wave propagation and plasticity of interstitial cells of Cajal in the small intestine of diabetic rats. Exp Physiol. 2011;96:1039–48.
Kawano K, Hirashima T, Mori S, et al. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–8.
Kim SJ, Park JH, Song DK, et al. Alterations of colonic contractility in long-term diabetic rat model. J Neurogastroenterol Motil. 2011;17:372–80.
Lorincz A, Redelman D, Horvath VJ, et al. Progenitors of interstitial cells of Cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–93.
Bardsley MR, Horvath VJ, Asuzu DT, et al. Kitlow stem cells cause resistance to Kit/platelet-derived growth factor alpha inhibitors in murine gastrointestinal stromal tumors. Gastroenterology. 2010;139:942–52.
Maneesh D, Hayashi Y, Gajdos GB, et al. Stem cells for murine interstitial cells of Cajal suppress cellular immunity and colitis via prostaglandin E2 secretion. Gastroenterology. 2015;148:978–90.
He CL, Soffer EE, Ferris CD, et al. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34.
Nakahara M, Isozaki K, Hirota S, et al. Deficiency of KIT positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–70.
Nielsen DS, Krich L, Buschard K, et al. Beyond genetics. Influence of dietary factors and gut microbiota on type 1 diabetes. FEBS Lett. 2014;588:4234–43.
Vaarala O. Human intestinal microbiota and type 1 diabetes. Curr Diab Rep. 2013;13:601–7.
Atkinson MA, Bluestone JA, Eisenbarth GS, et al. How does type 1 diabetes develop? The notion of homicide or β-cell suicide revisited. Diabetes. 2011;60:1370–9.
Wheway J, Mackay CR, Newton RA, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202:1527–38.
Gutierrez-Canas I, Juarraz Y, Santiago B, et al. VIP down-regulates TLR4 expression and TLR4-mediated chemokine production in human rheumatoid synovial fibroblasts. Rheumatology. 2006;45:527–32.
Barbara G, Stanghellini V, Brandi G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100:2560–8.
Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306–14.
Bagyánszki M, Bodi N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J Diabetes. 2012;3:80–93.
Bravo JA, Forsythe P, Chew MV. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A. 2011;108:16050–5.
Kunze WA, Mao YK, Wang B. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J Cell Mol Med. 2009;13:2261–70.
Iyer LM, Aravind L, Coon SL, et al. Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004;20:292–9.
Asano Y, Hiramoto T, Nishino R. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1288–95.
Sobko T, Huang L, Midtvedt T. Generation of NO by probiotic bacteria in the gastrointestinal Tract. Free Radic Biol Med. 2006;41:985–91.
Schicho R, Krueger D, Zeller F. Hydrogen sulfide is a novel prosecretory neuromodulator in the Guinea-pig and human colon. Gastroenterology. 2006;131:1542–52.
Wirth R, Bódi N, Maróti G, et al. Regionally distinct alterations in the composition of the gut microbiota in rats with streptozotocin-induced diabetes. PLoS One. 2014;9, e110440.
Izbéki F, Wittman T, Rosztóczy A, et al. Immediate insulin treatment prevents gut motility alterations and loss of nitrergic neurons in the ileum and colon of rats with streptozotocin-induced diabetes. Diabetes Res Clin Pract. 2008;80:192–8.
Bódi N, Talapka P, Poles P, et al. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316–26.
Vaarala O, Atkinson MA, Neu J. The “perfect storm” for type 1 diabetes. The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–62.
Yarandi SS, Srinivasan S. Diabetic gastrointestinal motility disorders and the role of enteric nervous system: current status and future directions. Neurogastroenterol Motil. 2014;26:611–24.
Lundgren O, Svanvik J, Jivegard L. Enteric nervous system. I. Physiology and pathophysiology of the intestinal tract. Dig Dis Sci. 1989;34:264–83.
Furness JB, Callaghan BP, Rivera LR, et al. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71.
Kempler P, Amarenco G, Freeman R, et al. Toronto Consensus Panel on Diabetic Neuropathy. Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev. 2011;27:665–77. The latest panel guideline on autonomic neuropathy and gastrointestinal complications.
Søfteland E, Brock C, Frøkjær JB, et al. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J Diabetes Complicat. 2014;28:370–7.
Cesario V, Di Rienzo TA, Campanale M, et al. Methane intestinal production and poor metabolic control in type I diabetes complicated by autonomic neuropathy. Minerva Endocrinol. 2014;39:201–7.
Ojetti V, Pitocco D, Scarpellini E, et al. Small bowel bacterial overgrowth and type 1 diabetes. Eur Rev Med Pharmacol Sci. 2009;13:419–23.
Zietz B, Lock G, Straub RH, et al. Small-bowel bacterial overgrowth in diabetic subjects is associated with cardiovascular autonomic neuropathy. Diabetes Care. 2000;23:1200–1.
Gatopoulou A, Papanas N, Maltezos E. Diabetic gastrointestinal autonomic neuropathy: current status and new achievements for everyday clinical practice. Eur J Intern Med. 2012;23:499–505.
El-Salhy M. The possible role of the gut neuroendocrine system in diabetes gastroenteropathy. Histol Histopathol. 2002;17:1153–61.
Phillips LK, Rayner CK, Jones KL, et al. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr Diab Rep. 2006;6:417–23.
Samsom M, Jebbink RJ, Akkermans LM, et al. Abnormalities of antroduodenal motility in type I diabetes. Diabetes Care. 1996;19:21–7.
Nguyen LA, Snape WJ. Clinical presentation and pathophysiology of gastroparesis. Gastroenterol Clin N Am. 2015;44:21–30.
Chandrasekharan B, Anitha M, Blatt R, et al. Colonic motor dysfunction in human diabetes is associated with enteric neuronal loss and increased oxidative stress. Neurogastroenterol Motil. 2011;23:131–8.
Bharucha AE, Low P, Camilleri M, et al. A randomised controlled study of the effect of cholinesterase inhibition on colon function in patients with diabetes mellitus and constipation. Gut. 2013;62:708–15.
Jung HK, Kim DY, Moon IH, et al. Colonic transit time in diabetic patients—comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265–72.
Søfteland E, Brock C, Frøkjær JB, et al. Rectal sensitivity in diabetes patients with symptoms of gastroparesis. J Diabetes Res. 2014;2014:784841.
Rosztóczy A, Róka R, Várkonyi T, et al. Regional differences in the manifestation of gastrointestinal motor disorders in type 1 diabetic patients with autonomic neuropathy. Z Gastroenterol. 2004;42:1295–300.
Valdovinos MA, Camilleri M, Zimmerman BR. Chronic diarrhea in diabetes mellitus: mechanisms and an approach to diagnosis and treatment. Mayo Clin Proc. 1993;68:691–702.
Mourad FH, Gorard D, Thillainayagam AV, et al. Effective treatment of diabetic diarrhea with somatostatin analogue, octreotide. Gut. 1992;33:1578–80.
Horváth VJ, Izbéki F, Lengyel C, et al. Diabetic gastroparesis: functional/morphologic background, diagnosis, and treatment options. Curr Diab Rep. 2014;14:527–33.
Bytzer P, Talley NJ, Jones MP, et al. Oral hypoglycaemic drugs and gastrointestinal symptoms in diabetes mellitus. Aliment Pharmacol Ther. 2001;15:137–42.
Ruiter R, Visser LE, van Herk-Sukel MP, et al. Lower risk of cancer in patients on metformin in comparison with those on sulfonylurea derivatives: results from a large population-based follow-up. Diabetes Care. 2012;35:119–24.
Noto H, Goto A, Tsujimoto T, et al. Cancer risk in diabetic patients treated with metformin: a systematic review. PLoS One. 2012;7, e33411.
Zhang ZJ, Zheng ZJ, Kan H, et al. Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes. Diabetes Care. 2011;34:2323–8.
Landman GWD, Kleefstra N, van Hateren KJJ, et al. Metformin associated with lower cancer mortality in type 2 diabetes. Diabetes Care. 2010;33:322–6.
Compliance with Ethics Guidelines
Conflict of Interest
Viktor József Horváth, Zsuzsanna Putz, Ferenc Izbéki, Anna Erzsébet Körei, László Gerő, Csaba Lengyel, Péter Kempler, and Tamás Várkonyi declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Microvascular Complications—Neuropathy
Rights and permissions
About this article
Cite this article
Horváth, V.J., Putz, Z., Izbéki, F. et al. Diabetes-Related Dysfunction of the Small Intestine and the Colon: Focus on Motility. Curr Diab Rep 15, 94 (2015). https://doi.org/10.1007/s11892-015-0672-8
Published:
DOI: https://doi.org/10.1007/s11892-015-0672-8