Abstract
Purpose of Review
This review summarizes the relevant literature on the use of total neoadjuvant therapy (TNT) for patients with locally advanced rectal cancer. It highlights the most notable literature published and briefly discusses future directions.
Recent Findings
Recent randomized trials evaluating TNT show improved rates of pathologic complete response and patient treatment tolerance with this approach.
Summary
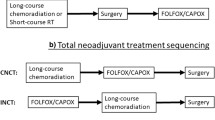
The rationale for TNT includes the poor patient tolerance of adjuvant chemotherapy and the persistent risk of distant disease in patients with locally advanced rectal cancer, despite improvements in local control. Randomized trials have focused on short-term pathologic endpoints. Ongoing phase 3 trials are evaluating long-term disease-related outcomes, allowing for a more thorough evaluation of this treatment paradigm. TNT may also facilitate organ preservation in appropriately selected patients.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
SEER Cancer Statistics [Available from: https://seer.cancer.gov/statfacts/html/colorect.html.
Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341(8843):457–60.
Peeters KC, Marijnen CA, Nagtegaal ID, Kranenbarg EK, Putter H, Wiggers T, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701.
Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811–20.
Swedish Rectal Cancer T, Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980–7.
Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol (R Coll Radiol). 2016;28(2):146–51.
Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(19):3109–16.
Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(16):2198–204.
• Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–90 Randomized control trial demonstrating no benefit to adjuvant fluorouracil-based chemotherapy in patient with rectal cancer after neoadjuvant radiotherapy (+/− concurrent chemotherapy).
Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol Off J Am Soc Clin Oncol. 2007;25(28):4379–86.
Sainato A, Cernusco Luna Nunzia V, Valentini V, De Paoli A, Maurizi ER, Lupattelli M, et al. No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): long term results of a randomized trial (I-CNR-RT). Radiother Oncol. 2014;113(2):223–9.
Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93(8):583–96.
Roh MS, Colangelo LH, O'Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(31):5124–30.
Frykholm GJ, Glimelius B, Pahlman L. Preoperative or postoperative irradiation in adenocarcinoma of the rectum: final treatment results of a randomized trial and an evaluation of late secondary effects. Dis Colon Rectum. 1993;36(6):564–72.
Gerard JP, Conroy T, Bonnetain F, Bouche O, Chapet O, Closon-Dejardin MT, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(28):4620–5.
Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15.
Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(16):1926–33.
Minsky BD. Short-course radiation versus long-course chemoradiation for rectal cancer: making progress. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(31):3777–8.
Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: trans-Tasman radiation oncology group trial 01.04. J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(31):3827–33.
Gastrointestinal Tumor Study G. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312(23):1465–72.
Breugom AJ, van Gijn W, Muller EW, Berglund A, van den Broek CB, Fokstuen T, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26(4):696–701.
Glynne-Jones R, Counsell N, Quirke P, Mortensen N, Maraveyas A, Meadows HM, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol. 2014;25(7):1356–62.
Torok JA, Palta M, Willett CG, Czito BG. Nonoperative management of rectal cancer. Cancer. 2016;122(1):34–41.
Methy N, Bedenne L, Conroy T, Bouche O, Chapet O, Ducreux M, et al. Surrogate end points for overall survival and local control in neoadjuvant rectal cancer trials: statistical evaluation based on the FFCD 9203 trial. Ann Oncol. 2010;21(3):518–24.
Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431–40.
•• Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–66 Phase 2 trial evaluating TNT (chemoradiation → chemotherapy) in patients with locally advanced rectal cancer, showing improvement in treatment compliance with neoadjuvant therapy. This trial also demonstrated increasing rates of pCR with additional cycles of neoadjuvant chemotherapy.
Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250(4):582–9.
Wolthuis AM, Penninckx F, Haustermans K, De Hertogh G, Fieuws S, Van Cutsem E, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19(9):2833–41.
Habr-Gama A, Perez RO, Proscurshim I, Nunes Dos Santos RM, Kiss D, Gama-Rodrigues J, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71(4):1181–8.
Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(31):3773–80.
•• Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Krynski J, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 x 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27(5):834–42 Randomized phase 3 trial comparing long-course chemoradiation to TNT utilizing short-course radiation therapy, showing no difference in the rate of R0 resection, local control, or disease-free survival. There was a survival benefit noted in the TNT arm at 3 years.
•• Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann Oncol. 2015;26(8):1722–8 Phase 2 trial comparing TNT to adjuvant chemotherapy, with no difference in disease-free or overall survival at 5 years but superior treatment compliance in the TNT arm.
•• Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C, et al. PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer. Ann Oncol. 2016;27(8):1557–65 Pooled analysis of the EXPERT (42) and EXPERT-C (43) trials.
Perez K, Safran H, Sikov W, Vrees M, Klipfel A, Shah N, et al. Complete neoadjuvant treatment for rectal Cancer: the Brown University oncology group CONTRE study. Am J Clin Oncol. 2017;40(3):283–7.
•• Habr-Gama A, Sabbaga J, Gama-Rodrigues J, Sao Juliao GP, Proscurshim I, Bailao Aguilar P, et al. Watch and wait approach following extended neoadjuvant chemoradiation for distal rectal cancer: are we getting closer to anal cancer management? Dis Colon Rectum. 2013;56(10):1109–17 Single arm prospective trial which showed improvement in the rate of clinical complete response with the addition of chemotherapy following chemoradiation for patients entering into a "watch-and-wait" or non-operative management approach.
Fernandez-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clini Oncol Off J Am Soc Clin Oncol. 2010;28(5):859–65.
Gao YH, An X, Sun WJ, Cai J, Cai MY, Kong LH, et al. Evaluation of capecitabine and oxaliplatin administered prior to and then concomitant to radiotherapy in high risk locally advanced rectal cancer. J Surg Oncol. 2014;109(5):478–82.
Gao YH, Lin JZ, An X, Luo JL, Cai MY, Cai PQ, et al. Neoadjuvant sandwich treatment with oxaliplatin and capecitabine administered prior to, concurrently with, and following radiation therapy in locally advanced rectal cancer: a prospective phase 2 trial. Int J Radiat Oncol Biol Phys. 2014;90(5):1153–60.
Zampino MG, Magni E, Leonardi MC, Petazzi E, Santoro L, Luca F, et al. Capecitabine initially concomitant to radiotherapy then perioperatively administered in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2009;75(2):421–7.
• Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11(3):241–8 Single arm prospective trial evaluating TNT in patients with locally advanced rectal cancer (neoadjuvant chemotherapy →chemoradiation → surgery → adjuvant chemotherapy), showing high rates of treatment compliance.
• Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol Off J Am Soc Clin Oncol. 2012;30(14):1620–7 Single arm prospective trial evaluating TNT in patients with locally advanced rectal cancer. Treatment paradigm the same as that in the EXPERT trial (42), with the addition of cetuximab. This trial also demonstrated high rates of treatment compliance.
Dipetrillo T, Pricolo V, Lagares-Garcia J, Vrees M, Klipfel A, Cataldo T, et al. Neoadjuvant bevacizumab, oxaliplatin, 5-fluorouracil, and radiation for rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82(1):124–9.
Dueland S, Ree AH, Groholt KK, Saelen MG, Folkvord S, Hole KH, et al. Oxaliplatin-containing preoperative therapy in locally advanced rectal Cancer: local response, toxicity and long-term outcome. Clin Oncol (R Coll Radiol). 2016;28(8):532–9.
Koeberle D, Burkhard R, von Moos R, Winterhalder R, Hess V, Heitzmann F, et al. Phase II study of capecitabine and oxaliplatin given prior to and concurrently with preoperative pelvic radiotherapy in patients with locally advanced rectal cancer. Br J Cancer. 2008;98(7):1204–9.
Marechal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525–30.
Nogue M, Salud A, Vicente P, Arrivi A, Roca JM, Losa F, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16(5):614–20.
Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Hogdall E, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23(10):2627–33.
Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr, Silva e Sousa AH Jr, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–7 discussion 7-8.
Habr-Gama A, Perez RO, Sabbaga J, Nadalin W, Sao Juliao GP, Gama-Rodrigues J. Increasing the rates of complete response to neoadjuvant chemoradiotherapy for distal rectal cancer: results of a prospective study using additional chemotherapy during the resting period. Dis Colon Rectum. 2009;52(12):1927–34.
Habr-Gama A, Perez RO, Sao Juliao GP, Proscurshim I, Fernandez LM, Figueiredo MN, et al. Consolidation chemotherapy during neoadjuvant chemoradiation (CRT) for distal rectal cancer leads to sustained decrease in tumor metabolism when compared to standard CRT regimen. Radiat Oncol. 2016;11:24.
Nilsson PJ, van Etten B, Hospers GA, Pahlman L, van de Velde CJ, Beets-Tan RG, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279.
Gollins S. A phase II single arm feasibility trial of neoadjuvant chemotherapy (NAC) with oxaliplatin/fluorouracil (OxMdG) then short-course preoperative radiotherapy (SCPRT) then immediate surgery in operable rectal cancer (ORC): COPERNICUS (NCT01263171). J Clin Oncol Off J Am Soc Clin Oncol. 2015;33 (supplement):Abstract 3609.
Ireland. TAoCoGBa. Operable rectal cancer: survey of current practice and proposed phase III trial. [Available from: http://www.acpgbi.org/uk/content/uploads/2014/07/CREATE-Survey-2014.pdf.
Borg C, Andre T, Mantion G, Boudghene F, Mornex F, Maingon P, et al. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI-defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann Oncol. 2014;25(11):2205–10.
Wong SJ, Moughan J, Meropol NJ, Anne PR, Kachnic LA, Rashid A, et al. Efficacy endpoints of radiation therapy group protocol 0247: a randomized, phase 2 study of neoadjuvant radiation therapy plus concurrent capecitabine and irinotecan or capecitabine and oxaliplatin for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2015;91(1):116–23.
Czito BG, Deming DA, Jameson GS, et al. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol. 2017 Jun;2(6):418–26.
George T, Yothers G, Hong T, et al. A phase II clinical trial platform utilizing total neoadjuvant therapy (TNT) in rectal cancer: Nrg-GI002. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34 (supplement):Abstract TPS3638.
Smith JJ, Chow OS, Gollub MJ, Nash GM, Temple LK, Weiser MR, et al. Organ preservation in rectal adenocarcinoma: a phase II randomized controlled trial evaluating 3-year disease-free survival in patients with locally advanced rectal cancer treated with chemoradiation plus induction or consolidation chemotherapy, and total mesorectal excision or nonoperative management. BMC Cancer. 2015;15:767.
Oncology AfCTi. PROSPECT: Chemotherapy alone or chemotherapy plus radiation therapy in treating patients with locally advanced rectal cancer undergoing surgery 2016 [Available from: https://clinicaltrials.gov/ct2/show/NCT01515787.
Glynne-Jones R, Hava N, Goh V, Bosompem S, Bridgewater J, Chau I, et al. Bevacizumab and combination chemotherapy in rectal cancer until surgery (BACCHUS): a phase II, multicentre, open-label, randomised study of neoadjuvant chemotherapy alone in patients with high-risk cancer of the rectum. BMC Cancer. 2015;15:764.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarah J. Stephens declares that she has no conflict of interest.
Christopher G. Willett declares that he has no conflict of interest.
Manisha Palta has received research support from Merck, honoraria from Oakstone CME and UpToDate, and has received compensation from Navigant for service as a consultant.
Brian G. Czito declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Additional information
This article is part of the Topical Collection on Radiation Therapy and Radiation Therapy Innovations in Colorectal Cancer
Rights and permissions
About this article
Cite this article
Stephens, S.J., Willett, C.G., Palta, M. et al. Total Neoadjuvant Therapy (TNT) in Rectal Cancer. Curr Colorectal Cancer Rep 14, 199–206 (2018). https://doi.org/10.1007/s11888-018-0415-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11888-018-0415-8