Abstract
Purpose of Review
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are commonly used anti-hypertensive medications in a number of clinical settings. They are often used interchangeably, but we pose the provocative question as to whether they should be. We review the literature to evaluate for any differences in efficacy between the two classes in order to determine if the greater side effects associated with angiotensin-converting enzyme inhibitors are offset by any advantageous effects on outcomes to warrant their use over angiotensin receptor blockers.
Recent Findings
In many clinical scenarios, the data supports similar efficacy between ACE inhibitors and ARBs, while in a minority of others, there are murky signals from previous trials that suggest ACE inhibitors may be better. However, when reviewing the literature in its entirety, and taking into account recently published pooled analysis and head to head trials, it is reasonable to conclude that ACE inhibitors and ARBs have similar efficacy. This is in contrast to data on adverse effects, which consistently favors the use of ARBs.
Summary
From the available data, it is reasonable to conclude that ACE inhibitors and ARBs have equal efficacy yet unequal adverse effects. It is in this context that we take the provocative stance that ACE inhibitors should not be used to treat hypertension.
Similar content being viewed by others
Introduction
Renin angiotensin aldosterone system (RAAS) plays a significant role in systemic blood pressure control. Several studies have shown its importance since renin was first discovered in 1898 by Tigerstedt and Bergman [1]. Subsequently, Goldblatt et al. [2] first demonstrated the development of hypertension in dogs when renal artery stenosis was experimentally induced. The compounds responsible for this were consequently isolated, initially named hypertensin, but eventually named angiotensin [3]. Eventually, through concerted efforts of various scientist groups, the whole cascade was progressively discovered. The first of the RAAS inhibitors to be introduced for widespread human use for hypertension were angiotensin-converting enzyme (ACE) inhibitors. The first ACE inhibitor approved by the FDA was captopril in 1981 [4]. Several ACE inhibitors have since been synthesized and are in clinical use currently. Angiotensin receptor blockers (ARBs) were discovered more than a decade later with the first ARB losartan being approved by the U.S. Food and Drug Administration in 1995 [5]. Both these classes of medications are used as anti-hypertensive agents in a variety of clinical conditions including essential hypertension, coronary artery disease, congestive heart failure, and chronic kidney disease. Over the past 40 years, a wealth of data has been produced from a number of well-designed trials regarding their impact on disease outcomes as well as their side effect profiles. Regarding side effects, the results disfavor ACE inhibitor use, because although the overall incidence is rare, angioedema-related deaths from these agents are significantly more frequent as compared to ARBs. It is in this context that if we conclude the benefits on outcomes are equivalent between ACE inhibitors and ARBs, then agents from these two classes should definitely not be considered interchangeable. The question therefore being posed is, should ACE inhibitors ever be used to treat hypertension?
Mechanisms of Action
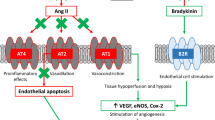
The therapeutic benefit of ACE inhibitors and ARBs is derived from their ability to disrupt RAAS, albeit the mechanism by which this occurs differs by class. The cascade of RAAS initiates the production of renin from the juxtaglomerular apparatus of the kidneys (Fig. 1). Renin then converts angiotensinogen produced in the liver to angiotensin I. Angiotensin I is subsequently cleaved by angiotensin-converting enzyme to angiotensin II. Angiotensin II acts via the AT1 receptor to directly promote vasoconstriction as well as sodium reabsorption in the proximal tubule of the kidney and via the AT2 receptor to stimulate aldosterone release from the adrenal cortex. Aldosterone independently acts in the distal nephron to regulate sodium and potassium homeostasis; in addition, aldosterone promotes end-organ fibrosis in the kidneys, heart, and vasculature, among other organ systems [6]. ACE inhibitors block the activity of angiotensin-converting enzyme, thereby preventing the conversion of angiotensin I to angiotensin II. Inhibition of this enzyme also disrupts the breakdown of bradykinin, which in turn promotes vasodilation through the kinin-kallikrein-bradykinin system. Elevated bradykinin is implicated in the pathogenesis of ACE inhibitor-induced cough and angioedema. Alternatively, ARBs directly inhibit the binding of angiotensin II to AT1 receptors. Unlike ACE inhibitors, ARBs do not significantly affect bradykinin levels, and therefore, cough and angioedema are much less common.
ACE Inhibitor Versus ARB Efficacy for BP Lowering
Drugs that inhibit RAAS were originally designed for the purpose of lowering blood pressure. Given the mechanisms by which ACE inhibitors and ARBs reduce blood pressure are unique for each class, significant research efforts have focused on understanding their differences. The ONTARGET trial directly compared the blood pressure lowering efficacy of ramipril at a dose of 10 mg daily with that of telmisartan at a dose of 80 mg daily [7]. The mean blood pressure reduction at 6 weeks was − 7.5/− 5.0 mmHg in the group receiving telmisartan and − 6.4/− 4.3 mmHg in the group receiving ramipril, demonstrating that telmisartan was non-inferior to ramipril for lowering blood pressure in patients with vascular disease or diabetes. In another head to head study, fixed dose olmesartan 40 mg in combination with amlodipine 10 mg demonstrated superior central systolic blood pressure lowering and 24 h ambulatory blood pressure lowering effects when compared to fixed dose perindopril 8 mg in combination with amlodipine 10 mg [8]. Additionally, the newer ARB agent azilsartan was shown to be superior to ramipril for blood pressure lowering efficacy [9]. In elderly individuals (65–89 years old), olmesartan had a greater effect on lowering 24 h ambulatory blood pressure than did ramipril [10]. Finally, in a systematic review of 47 head to head studies that reported comparative blood pressure effects between ACE inhibitors and ARBs, 37 demonstrated no difference, 2 favored ACE inhibitors, and 8 favored ARBs [11]. There was no overall significant difference between ACE inhibitors and ARBs in the pooled analysis. Together, these data overwhelmingly conclude that the anti-hypertensive effects of ARBs are not inferior to ACE inhibitors.
ACE Inhibitor Versus ARB on Clinical Outcomes Areas of More Controversy
Reducing Cardiovascular Events
The ARB myocardial infarction paradox theorizes that not only are ARBs inferior to ACE inhibitors for reducing cardiovascular events but ARBs may actually increase these risks [12]. This theory originated in 2004 following the release of results from the VALUE trial, which compared reductions in cardiovascular morbidity and mortality between valsartan and amlodipine [13]. The group treated with valsartan had a 19% relative increase in myocardial infarctions as compared to those treated with amlodipine (p = 0.02). Similar findings were reflected in previous placebo-controlled ARB trials at that time. The CHARM-alternative trial reported a 36% relative increase in myocardial infarctions with candesartan versus placebo in congestive heart failure (CHF) population, and the SCOPE trial demonstrated a non-significant trend toward higher myocardial infarctions in elderly subjects with hypertension who received candesartan versus placebo [14, 15]. These results were in stark contrast to previous placebo-controlled trials with ACE inhibitors that demonstrated consistent cardioprotective benefits in various populations. More recently, a number of indirect comparisons with pooled analysis of several randomized placebo control trials have been done to further elucidate whether ACE inhibitors and ARBs have differing effects on the risk of myocardial infarction [16,17,18,19]. These data also suggest a superior benefit of ACE inhibitors over ARBs for the reduction of myocardial infarction and/or cardiovascular death.
However, while the theory of the ARB myocardial infarction paradox has persisted, it is important to recognize that the abovementioned data are only part of the story, and despite it, there is still good reason to believe that ACE inhibitors and ARBs are in fact similar in their impact on myocardial infarction. First, it is important to recognize that a generational gap exists between many of the ACE inhibitor placebo-controlled trials versus the ARB placebo-controlled trials. Most of the ACE inhibitor trials occurred prior to the year 2000, and most of the ARB trials occurred after the year 2000. This results in important differences between the standard clinical practices in place for primary and secondary prevention of cardiovascular events, namely statins and effective blood pressure management. Due to this, event rates in a study population from the 1980s and 1990s are likely to be vastly different from those in the 2000s and 2010s. This was demonstrated in the meta-analysis by Bangalore, which reported placebo-controlled trials conducted before the year 2000 (which were most of the ACE inhibitor trials) had higher event rates in the control group as compared to those conducted after the year 2000 (which were most of the ARB trials) (event rate 10.5% pre-2000 versus event rate 5.0% post-2000) [16]. The authors conducted a sensitivity analysis restricting the comparison of placebo-controlled ACE inhibitor and placebo-controlled ARB studies only to those conducted after the year 2000, and this showed similar cardiovascular event outcomes between the two anti-hypertensive classes. More importantly, we now have well-designed head to head randomized trials comparing the effects of myocardial infarction and cardiovascular death from ACE inhibitors and ARBs. The largest of these is the ONTARGET trial where more than 17,000 subjects were randomized to receive either telmisartan or ramipril as monotherapy [7]. In this study, there was no difference in the incidence of myocardial infarction or cardiovascular death observed between the two agents. Finally, in the previously mentioned Bangalore paper, a separate meta-analysis of the only head to head trials between ACE inhibitors and ARBs also showed no difference in the risk for myocardial infarction or cardiovascular death between the two classes [16].
While debate still exists regarding the differences of ACE inhibitors versus ARBs on the reduction of myocardial infarction and cardiovascular death, we feel the complete story of these data concludes there is truly no difference between the two classes.
First-Line Therapy in Heart Failure with Left Ventricular Dysfunction
The role of ARBs as first-line therapy in the treatment of heart failure with left ventricular dysfunction is also controversial. Both European and American guidelines state that ARB therapy in heart failure with reduced ejection fraction (HFrEF) should be reserved for those who are either intolerant of ACE inhibitors or who remain symptomatic despite ACE inhibitor therapy, and in the latter scenario, subjects with HFrEF should be treated with an ARB in the form of an angiotensin receptor neprilysin inhibitor [20, 21]. These guidelines stem from differences in the robustness of available data on the all-cause mortality benefit from treatment with ACE inhibitors versus treatment with ARBs in heart failure trials.
In patients with left ventricular dysfunction, ACE inhibitors have consistently shown significant reductions in both cardiovascular morbidity and mortality as well as all-cause mortality in placebo-controlled trials [22,23,24,25]. Because of these known mortality benefits of ACE inhibitor treatment in HFrEF, the subsequently undertaken heart failure studies using ARBs could not ethically be designed as placebo control trials in which ACE inhibitor therapy was excluded outright in the placebo group. For this reason, there is much less available data assessing the all-cause mortality benefit of ARBs as compared to placebo in subjects not taking an ACE inhibitor. However, the limited data that does exist argues that the benefit is equal. The CHARM-alternative trial, which compared candesartan to placebo in patients with HFrEF who were intolerant to ACE inhibitors, reported a significant reduction in cardiovascular mortality, CHF hospitalizations, and all-cause mortality similar to those seen with previous ACE inhibitor trials [15]. Additionally, in randomized trials designed to compare ACE inhibitors and ARBs head to head in patients with HFrEF, the impact on all-cause mortality, as well as cardiovascular mortality and morbidity, was similar [26, 27]. Finally, in a recently published network meta-analysis of 57 randomized control trials conducted over the past 30 years, a similar impact on all-cause mortality for ACE inhibitor and ARB therapies was reported [28]. Therefore, despite the limited availability of data for all-cause mortality reduction with ARBs in HFrEF, there still exists enough credible evidence to conclude that ARB agents provide a similar benefit as compared to ACE inhibitors for all-cause mortality, as well as other outcomes, in HFrEF. It is therefore reasonable to conclude these agents could be used interchangeably as first-line therapy solely based on their efficacy to impact outcomes.
ACE Inhibitors Versus ARBs on Clinical Outcomes Areas of More Consensus
Renal Outcomes
Robust data support the benefit of using either ACE inhibitors or ARBs on improving renal outcomes in patients with chronic kidney disease with proteinuria. In those with diabetes and either microalbuminuria with preserved glomerular filtration rate or diabetes with chronic kidney disease (CKD), ACE inhibitors have demonstrated benefit in reducing proteinuria, slowing progression of the disease, and delaying the time until reaching end-stage kidney disease in multiple studies [29,30,31,32,33]. Angiotensin receptor blockers have shown similar benefits on renal outcomes in diabetics, albeit, the majority of these data are in type 2 diabetics [34,35,36]. In non-diabetics with CKD and proteinuria, improvement in renal outcomes has been demonstrated with ACE inhibitors; however, studies with ARBs in this population are notably lacking [37, 38]. In head to head studies, ACE inhibitors and ARBs had a similar benefit on renal outcomes in those with CKD [39, 40]. Overall, there is no compelling evidence to suggest that there is superiority in renal outcomes in ACE inhibitors compared to ARBs.
Stroke Prevention
For those subjects who have previously had an ischemic stroke or transient ischemic attack, blood pressure reduction with anti-hypertensive medications is critical for secondary prevention. Data from the HOPE study showed that ramipril is beneficial for secondary prevention of stroke based on subgroup analysis [41]. The PROGRESS study showed the combination of the ACE inhibitor perindopril plus the thiazide diuretic indapamide to be more effective at blood pressure lowering and prevention of recurrent stroke than perindopril alone [42]. Meanwhile, data from the ONTARGET study showed no difference in primary stroke prevention between telmisartan and ramipril [7]. There is a dearth of data available to directly compare the impact of ACE inhibitors versus ARBs on secondary stroke prevention, but overall, the consensus based on extrapolation from previous trials is that ACE inhibitors and ARBs are equal in this regard. This sentiment is also reflected in the 2017 ACC/AHA/HFSA Hypertension guidelines [43].
ACE Inhibitors Versus ARBs Adverse Events
The most common side effect in patients using ACE inhibitors is cough. Although not conclusive, it is thought to be induced by high levels of bradykinins due to inhibition of its degradation to inactive peptides. The reported incidence of cough with ACE inhibitors varies widely in different studies but is generally accepted to be around 5–20% [44]. The incidence is higher among Asian populations. A meta-analysis by Matchar et al. showed that among randomized controlled trials in the analysis, the rate of cough in ACE inhibitors group was 9.9% while ARBs group had a rate of 3.2% (absolute risk difference of 6.7%) [11]. Angioedema is another complication of RAAS blockers. Although it occurs with much less frequency, it is a much more serious complication. In a meta-analysis by Makani et al., the incidence of angioedema with ACE inhibitors was 0.3% (95% CI 0.28–0.32), while the incidence with ARBs was 0.13% (95% CI 0.08–0.19) [45]. The risk of angioedema was twice as high in ACE inhibitors as compared to ARBs (RR 2.2, 95% CI 1.5–3.34, p < 0.0001). There was no statistically significant difference between ARBs and placebo. Other reported side effects with RAAS blockers include hyperkalemia and acute kidney injury (AKI); while these are rare in patients with normal renal function, the incidence progressively increases in patients with advancing stages of kidney disease. The burden of these adverse events is equal between ACE inhibitors and ARBs.
Cost and Availability
One of the main reasons cited in favor of ACEi use in the past was the cost difference that existed. In the 1990s, ACEi had already become generic while ARBs were just introduced and were much more expensive. As previously mentioned, losartan was the first ARB to be approved by the FDA in 1995. It is also the first ARB to be made generic in 2010. Since then, the majority of ARBs have been made available as generic formulations as well and the prices have significantly dropped. Although the prices vary quite significantly even within ACE inhibitors and ARBs, and whether they are generic or branded, the most used generic preparations are comparable in price between these groups and are equally affordable. Hence, currently, when considering only the price of these medications in the generic market, there is no clear winner.
In general, the availability of ACE inhibitors and ARBs is similar in the USA and other developed countries. However, it is worth mentioning that in 2018 and 2019, a series of recalls involving three ARB agents, valsartan, losartan, and irbesartan, did have a temporary impact on the supply of these drugs [46]. The basis of these recalls involved the presence of probable carcinogens that were introduced through manufacturing processes from two factories in China and India. Production of valsartan, losartan, and irbesartan from other manufacturers did not contain the identified carcinogens, and therefore, the recall did not remove the availability of these drugs from the market in their entirety. In total, about one-sixth of drug companies producing ARBs in the USA were impacted.
ACE Inhibitor and ARB Use During the Current COVID-19 Pandemic: Where Do We Stand?
It is now well established that angiotensin-converting enzyme 2 (ACE2) acts a receptor for SARS-COV2, the virus responsible for COVID-19, and enables its entry into cells [47]. ACE2 is a homologous enzyme to ACE and converts angiotensin II into angiotensin 1–7 which has vasodilatory properties. Several observational studies during the pandemic have noted that patients with hypertension are more susceptible to COVID-19 infections and its complications including severe respiratory illness, ICU stay, and death [48, 49]. Given what is known about viral entry into cells, an indirect conclusion was drawn that RAAS inhibitors can increase susceptibility to COVID-19 infection by increasing ACE2 levels. However, many studies involving ACE inhibitors and ARBs have shown inconsistent results with ACE2 mRNA and enzyme level expression [50, 51]. Direct evidence on the infectivity of the virus and its complications in patients taking RAAS blockers is sparse. On the contrary, based on data from a non-COVID-related lung injury, it is postulated that elevated ACE2 levels during COVID-19 infection could be protective of the lung parenchyma, thus preventing more serious illness [52,53,54]. This has led various organizations including AHA to recommend against discontinuation of ACE inhibitors or ARBs when diagnosed with COVID-19.
Conclusion
Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers have resulted in significant improvements in outcomes related to congestive heart failure, cardiovascular disease, diabetes, stroke, and kidney disease. It goes without saying that the widespread use of these therapies has saved a countless number of lives. However, as we look toward the future, it is important to ask how we can continue to maximize the benefits and minimize the risk of therapies related to RAAS inhibition. It appears we may now be at a critical point in which enough data has amassed to allow us to conclude that the impact on clinical outcomes between ACE inhibitors and ARBs are equivalent, but the risk associated with these agents clearly favors using ARBs.
The loudest arguments against equivalency in outcomes between ACE inhibitors and ARBs have surrounded concerns regarding higher myocardial infarction risks with ARBs, the so-called ARB myocardial paradox. However, when the data is critically reviewed in the context of the different event rates that existed due to the generational gap between placebo control ACE inhibitor and ARB trials, in addition to looking at more contemporary data from head to head studies, these arguments do not hold.
Regarding outcomes in HFrEF, there are similar gaps between trials conducted with ACE inhibitors versus ARBs. As a result, there is less robust data regarding the improvement in all-cause mortality with ARBs in HFrEF, despite there being similarities in reductions of cardiovascular death and heart failure hospitalizations. However, when closely examining the few trials that directly address this, including head to head studies and contemporary meta-analysis, the data concludes equal efficacy in all-cause mortality between the two classes in subjects with HFrEF.
On the other hand, the literature consistently demonstrates a small but statistically significant worse side effect profile with ACE inhibitors compared to ARBs. While the overall risk of death from angioedema with an ACE inhibitor is not high, it is high enough that it should still discourage their use over ARBs.
We therefore conclude that the use of ARBs for RAAS inhibition is a superior therapeutic strategy over the use of ACE inhibitors, and clinical practice patterns should shift in this direction.
References
Tigerstedt R, Bergman PQ. Niere und Kreislauf1. Skandinavisches Archiv Für Physiologie. 1898;8(1):223–71. https://doi.org/10.1111/j.1748-1716.1898.tb00272.x.
Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : I. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59(3):347–79. https://doi.org/10.1084/jem.59.3.347.
Schwarz H, Bumpus FM, Page IH. Synthesis of a biologically active octapeptide similar to natural isoleucine angiotonin octapeptide1. J Am Chem Soc. 1957;79(21):5697–703. https://doi.org/10.1021/ja01578a030.
Ram CVS. Captoril. Arch Intern Med. 1982;142(5):914–6. https://doi.org/10.1001/archinte.1982.00340180072016.
Burnier M, Brunner HR. Angiotensin II receptor antagonists. Lancet. 2000;355(9204):637–45. https://doi.org/10.1016/S0140-6736(99)10365-9.
Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9(8):459–69. https://doi.org/10.1038/nrneph.2013.110.
Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–59. https://doi.org/10.1056/NEJMoa0801317.
Ruilope L, Schaefer A. The fixed-dose combination of olmesartan/amlodipine was superior in central aortic blood pressure reduction compared with perindopril/amlodipine: a randomized, double-blind trial in patients with hypertension. Adv Ther. 2013;30(12):1086–99. https://doi.org/10.1007/s12325-013-0076-6.
Bönner G, Bakris GL, Sica D, Weber MA, White WB, Perez A, et al. Antihypertensive efficacy of the angiotensin receptor blocker azilsartan medoxomil compared with the angiotensin-converting enzyme inhibitor ramipril. J Hum Hypertens. 2013;27(8):479–86. https://doi.org/10.1038/jhh.2013.6.
Omboni S, Malacco E, Mallion JM, Volpe M, Zanchetti A, Group S. Twenty-four hour and early morning blood pressure control of olmesartan vs. ramipril in elderly hypertensive patients: pooled individual data analysis of two randomized, double-blind, parallel-group studies. J Hypertens. 2012;30(7):1468–77. https://doi.org/10.1097/HJH.0b013e32835466ac.
Matchar DB, McCrory DC, Orlando LA, Patel MR, Patel UD, Patwardhan MB, et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers for treating essential hypertension. Ann Intern Med. 2008;148(1):16–29. https://doi.org/10.7326/0003-4819-148-1-200801010-00189.
Strauss MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation. 2017;135(22):2088–90. https://doi.org/10.1161/CIRCULATIONAHA.117.026112.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–31. https://doi.org/10.1016/S0140-6736(04)16451-9.
Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21(5):875–86. https://doi.org/10.1097/00004872-200305000-00011.
Granger CB, McMurray JJ, Yusuf S, Held P, Michelson EL, Olofsson B, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. 2003;362(9386):772–6. https://doi.org/10.1016/S0140-6736(03)14284-5.
Bangalore S, Fakheri R, Toklu B, Ogedegbe G, Weintraub H, Messerli FH. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers in patients without heart failure? Insights from 254,301 patients from randomized trials. Mayo Clin Proc. 2016;91(1):51–60. https://doi.org/10.1016/j.mayocp.2015.10.019.
Savarese G, Costanzo P, Cleland JG, Vassallo E, Ruggiero D, Rosano G, et al. A meta-analysis reporting effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in patients without heart failure. J Am Coll Cardiol. 2013;61(2):131–42. https://doi.org/10.1016/j.jacc.2012.10.011.
Cheng J, Zhang W, Zhang X, Han F, Li X, He X, et al. Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med. 2014;174(5):773–85. https://doi.org/10.1001/jamainternmed.2014.348.
Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs--overview and meta-analyses. J Hypertens. 2015;33(2):195–211. https://doi.org/10.1097/HJH.0000000000000447.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776–803. https://doi.org/10.1016/j.jacc.2017.04.025.
Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN, Investigators S. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302. https://doi.org/10.1056/NEJM199108013250501.
Group CTS. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med. 1987;316(23):1429–35. https://doi.org/10.1056/NEJM198706043162301.
Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333(25):1670–6. https://doi.org/10.1056/NEJM199512213332503.
Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327(10):669–77. https://doi.org/10.1056/NEJM199209033271001.
Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355(9215):1582–7. https://doi.org/10.1016/s0140-6736(00)02213-3.
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349(20):1893–906. https://doi.org/10.1056/NEJMoa032292.
Burnett H, Earley A, Voors AA, Senni M, McMurray JJ, Deschaseaux C, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10(1). https://doi.org/10.1161/CIRCHEARTFAILURE.116.003529.
Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. https://doi.org/10.1056/NEJM199311113292004.
Mathiesen ER, Hommel E, Giese J, Parving HH. Efficacy of captopril in postponing nephropathy in normotensive insulin dependent diabetic patients with microalbuminuria. BMJ. 1991;303(6794):81–7. https://doi.org/10.1136/bmj.303.6794.81.
Laffel LM, McGill JB, Gans DJ. The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. Am J Med. 1995;99(5):497–504. https://doi.org/10.1016/s0002-9343(99)80226-5.
Ahmad J, Siddiqui MA, Ahmad H. Effective postponement of diabetic nephropathy with enalapril in normotensive type 2 diabetic patients with microalbuminuria. Diabetes Care. 1997;20(10):1576–81. https://doi.org/10.2337/diacare.20.10.1576.
Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med. 1998;128(12 Pt 1):982–8. https://doi.org/10.7326/0003-4819-128-12_part_1-199806150-00004.
Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870–8. https://doi.org/10.1056/NEJMoa011489.
Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345(12):851–60. https://doi.org/10.1056/NEJMoa011303.
Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. https://doi.org/10.1056/NEJMoa011161.
Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, et al. Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet. 1999;354(9176):359–64. https://doi.org/10.1016/S0140-6736(98)10363-X.
Wright JT, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–31. https://doi.org/10.1001/jama.288.19.2421.
Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med. 2004;351(19):1952–61. https://doi.org/10.1056/NEJMoa042274.
Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–53. https://doi.org/10.1016/S0140-6736(08)61236-2.
Bosch J, Yusuf S, Pogue J, Sleight P, Lonn E, Rangoonwala B, et al. Use of ramipril in preventing stroke: double blind randomised trial. BMJ. 2002;324(7339):699–702. https://doi.org/10.1136/bmj.324.7339.699.
Group PC. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033–41. https://doi.org/10.1016/S0140-6736(01)06178-5.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–248. https://doi.org/10.1016/j.jacc.2017.11.006.
Israili ZH, Hall WD. Cough and Angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: a review of the literature and pathophysiology. Ann Intern Med. 1992;117(3):234–42. https://doi.org/10.7326/0003-4819-117-3-234.
Makani H, Messerli FH, Romero J, Wever-Pinzon O, Korniyenko A, Berrios RS, et al. Meta-analysis of randomized trials of angioedema as an adverse event of renin–angiotensin system inhibitors. Am J Cardiol. 2012;110(3):383–91. https://doi.org/10.1016/j.amjcard.2012.03.034.
Byrd JB, Chertow GM, Bhalla V. Hypertension hot potato - anatomy of the angiotensin-receptor blocker recalls. N Engl J Med. 2019;380(17):1589–91. https://doi.org/10.1056/NEJMp1901657.
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. https://doi.org/10.1016/j.cell.2020.02.052.
Wu C, Chen X, Cai Y, Xia Ja, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Internal Medicine 2020. https://doi.org/10.1001/jamainternmed.2020.0994.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. https://doi.org/10.1016/S0140-6736(20)30566-3.
Hamming I, van Goor H, Turner AJ, Rushworth CA, Michaud AA, Corvol P, et al. Differential regulation of renal angiotensin-converting enzyme (ACE) and ACE2 during ACE inhibition and dietary sodium restriction in healthy rats. Exp Physiol. 2008;93(5):631–8. https://doi.org/10.1113/expphysiol.2007.041855.
Soler MJ, Ye M, Wysocki J, William J, Lloveras J, Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296(2):F398–405. https://doi.org/10.1152/ajprenal.90488.2008.
Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Critical care (London, England). 2017;21(1):234. https://doi.org/10.1186/s13054-017-1823-x.
Gu H, Xie Z, Li T, Zhang S, Lai C, Zhu P, et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci Rep. 2016;(6):19840. https://doi.org/10.1038/srep19840.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–6. https://doi.org/10.1038/nature03712.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jeffrey M. Turner reports personal fees from Tricida. Ravi Kodali declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Hypertension
Rights and permissions
About this article
Cite this article
Turner, J.M., Kodali, R. Should Angiotensin-Converting Enzyme Inhibitors ever Be Used for the Management of Hypertension?. Curr Cardiol Rep 22, 95 (2020). https://doi.org/10.1007/s11886-020-01352-8
Published:
DOI: https://doi.org/10.1007/s11886-020-01352-8