Abstract
Prior work has reported acute associations between ST-elevation myocardial infarction (STEMI) and short-term increases in airborne particulate matter. Subsequently, the association between STEMI and hourly measures of Delta-C (marker of woodsmoke) and black carbon (marker of traffic pollution) measured at a central site in Rochester, NY, were examined, but no association was found. Therefore, land use regression estimates of Delta-C and black carbon concentrations at each patient’s residence were developed for 246 STEMI patients treated at the University of Rochester Medical Center during the winters of 2008–2012. Using case-crossover methods, the rate of STEMI associated with increased Delta-C and BC concentration on the same and previous 3 days was estimated after adjusting for 3-day mean temperature and relative humidity. Non-statistically significant increased rates of STEMI associated with interquartile range increases in concentrations of BC in the previous 2 days (1.10 μg/m3; OR = 1.12; 95% CI 0.93, 1.35) and Delta-C in the previous 3 days (0.43 μg/m3; OR = 1.16; 95% CI 0.96, 1.40) were found. Significantly increased rates of STEMI associated with interquartile range increases in concentrations of BC (1.23 μg/m3; OR = 1.04; 95% CI = 0.87, 1.24) or Delta-C (0.40 μg/m3; OR = 0.94; 95% CI = 0.85, 1.09) on the same day were not observed likely due, in part, to temporal misalignment. Therefore, sophisticated spatial-temporal models will be needed to minimize exposure error and bias by better predicting concentrations at individual locations for individual hours, especially for outcomes with short-term responses to air pollution (< 24 h).
Similar content being viewed by others
Introduction
Short-term increases in ambient particulate matter (PM) concentrations over hours and days have been associated with various manifestations of cardiovascular disease, including myocardial infarction (MI) (Mustafic et al. 2012; Gardner et al. 2014). Previously, Gardner et al. (2014) observed a significant 18% increase in the rate of ST-elevation myocardial infarction (STEMI), but not non-ST-elevation myocardial infarction (NSTEMI), associated with each 7.1 μg/m3 increase in fine particulate pollution (< 2.5 μm diameter; PM2.5) concentrations in the previous hour among patients in Monroe County, NY. Others have subsequently reported triggering of STEMI, but not NSTEMI, by short-term increases in PM2.5 concentrations (Pope et al. 2015; Zhang et al. 2016). However, it is unknown which PM2.5 constituent(s)/source(s) trigger STEMI.
Previously, Wang et al. (2012a, b) estimated that up to 30% of wintertime PM2.5 is contributed by woodsmoke in Rochester, NY. Therefore, Evans et al. (2017) examined whether increased hourly concentrations of source-specific PM2.5, including Delta-C (marker of woodsmoke) (Wang et al. 2011a) and black carbon (marker of traffic pollution) (Suglia et al. 2008), measured at a single, central, monitoring station in Rochester in the previous 1, 12, 24, 48, 72, and 96 h were associated with an increased rate of STEMI. However, neither pollutant was associated with an increased rate of STEMI in the previous 96 h (Evans et al. 2017). In that analysis, all study subjects’ pollutant concentrations were taken from the same monitoring station no matter how far they lived from it, resulting in exposure error and likely effect underestimation (Evans et al. 2017). This exposure error may be fairly substantial for Delta-C and black carbon, which have greater spatial variability in concentration than PM2.5 (Larson et al. 2007; Su et al. 2007; Wang et al. 2011b). Wang et al. (2011b) measured BC at a number of locations in Rochester and concluded that one central monitoring site may not adequately represent the actual residential wood combustion particle exposure over the whole urban area. Thus, this exposure error could induce substantial attenuation of the estimated associations between STEMI and these pollutants. Use of central site measured concentrations alone may not be sufficient to observe air pollution-mediated health effects if they do exist.
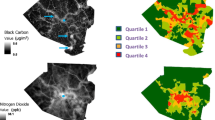
Thus, a land use regression (LUR) model was developed to predict daily concentrations of black carbon and Delta-C at the residence of each STEMI patient during the study period, to provide specific estimates of subjects’ actual exposures to these pollutants (Su et al. 2015). Using these daily LUR estimates of black carbon and Delta-C concentrations, and the same data set of STEMI events, it was hypothesized that increased concentrations of black carbon and Delta-C on the same day as the STEMI would be associated with an increased rate of STEMI.
Methods
Study population
The study population was described previously by Evans et al. (2017). Briefly, patients treated at the Cardiac Catheterization Laboratory (Cath Lab) at the University of Rochester Medical Center in Rochester, NY, for STEMI between November 1 and April 30 from 2008 to 2012, who were Monroe County, NY residents, and for whom symptom onset time was recorded were included in the analysis (n = 246). Using American College of Cardiology/American Heart Association guidelines at the time of Cath Lab admittance, STEMI was diagnosed as ST segment elevation on the electrocardiogram of > 1 mm in ≥ 2 contiguous precordial leads, or ≥ 2 adjacent limb leads, or new or presumed-new left bundle branch block in the presence of angina or anginal equivalent. If a patient was admitted for multiple MIs during the study, each STEMI was included in the analysis if it was at least 3 days after the previous MI. Information on patient demographic and clinical characteristics, including smoking, history of MI, and other comorbidities (peripheral artery disease, heart failure, diabetes, dyslipidemia, and hypertension) were also retained. All study activities were approved by the University of Rochester Research Subjects Review Board.
Air pollution and meteorology measurements
Black carbon, Delta-C, and particulate matter < 2.5 μm (PM2.5) concentrations, temperature, and relative humidity measured between November 1, 2008 and April 30, 2012 at a New York State Department of Environmental Conservation (DEC) site in Rochester, NY, were used to drive the model predictions. Using a two-wavelength (370 and 880 nm) aethalometer (Model AE22, Magee Scientific, Inc., Berkeley, CA), black carbon was estimated using the 880-nm measurements, with Delta-C calculated as the difference between the 370-nm and 880-nm measurements. Delta-C is a surrogate for woodsmoke because it was highly correlated with levoglucosan (Wang et al. 2011a), the compound commonly used as a marker of wood combustion particles (Simoneit 2002). Hourly PM2.5 concentrations were measured continuously using a tapered element oscillating microbalance (TEOM; model 1400ab, ThermoFisher, Franklin, MA). Ambient temperature and relative humidity were measured in 5-min intervals at the DEC site and provided as hourly averages. The residential addresses of each study subject were geocoded (ArcGis Version-10.3.1.©Esri, Redlands, CA), and daily black carbon and Delta-C concentrations were estimated using the land use regression model developed for the cold period (November 1 to April 30) in Monroe County, NY (Su et al. 2015).
Study design and statistical analyses
The time-stratified case-crossover design (Levy et al. 2001) used previously in studies of ambient air pollution and MI (Mustafic et al. 2012; Gardner et al. 2014; Pope et al. 2015; Evans et al. 2017) was used in this study. Mean concentrations of Delta-C, BC, temperature, and relative humidity were calculated for the same day (lag day 0) and previous 2 days (lag days 0–1), 3 days (lag days 0–2), and 4 days (lag days 0–3). Conditional logistic regression models were used to estimate the rate (and 95% confidence intervals) of STEMI associated with interquartile range (IQR) increases in pollutant concentrations during each lag period, adjusting for the 3-day mean temperature and relative humidity using natural splines (2 degrees of freedom). SAS version 9.3 (SAS Institute, Inc., Cary, NC) was used to construct all data sets and perform descriptive analyses, and R version 3.0.1 (R Foundation for Statistical Computing, Vienna, Austria) was used for all conditional logistic regression models.
Results
Most patients (52%) were between 50 and 69 years old (mean age ± standard deviation = 61.8 ± 15.5 years). Subjects were primarily male (68%) and non-Hispanic white (80%). Approximately one third were smokers (37%) and 76% were either overweight or obese (BMI ≥ 25 kg/m2; Table 1). The median and mean ± standard deviation of daily black carbon concentrations on control days during the study period were 0.69 μg/m3 and 1.10 ± 1.25 μg/m3. For Delta-C, they were 0.10 μg/m3 and 0.34 ± 0.64 μg/m3. For case periods, the median and mean ± standard deviation of daily black carbon concentrations were 0.68 μg/m3 and 1.10 ± 1.29 μg/m3, while for Delta-C, they were 0.11 μg/m3 and 0.31 ± 0.57 μg/m3. Daily LUR-estimated black carbon and Delta-C were minimally correlated (Spearman r = 0.32). For comparison, the mean ± standard deviation ambient PM2.5 concentrations at the Rochester monitoring station were 7.8 ± 4.1 μg/m3 for case periods and 7.8 ± 4.0 μg/m3 for control periods.
Although imprecise and not statistically significant, increased rates of STEMI were associated with interquartile range increases in concentrations of black carbon in the previous 2 days (1.10 μg/m3; OR = 1.12; 95% CI 0.93, 1.35) and Delta-C in the previous 3 days (0.43 μg/m3; OR = 1.16; 95% CI 0.96, 1.40). No increased rates of STEMI associated with Delta-C or black carbon concentrations on the same day, as hypothesized, were observed (Table 2). In a sensitivity analysis using the land use regression estimated Delta-C and black carbon concentrations averaged over the same days, and averaged over a 500-m radius around the location of each residence (Su et al. 2015), similar size effects were found (Table 2). In a second sensitivity analysis, we re-ran the same models, including the ambient PM2.5 concentration for the same lag time to determine if any effect of BC or Delta-C was independent of total fine particle mass. However, after adjustment for PM2.5, there was little change in the rate of STEMI associated with each IQR increase in Delta-C in the previous 3 days (OR = 1.16, 95% CI = 0.96, 1.40) or black carbon in the previous 2 days (OR = 1.12; 95% CI = 0.93, 1.35).
Discussion
In a case-crossover study examining the triggering of ST-elevation myocardial infarction by short-term increases in daily ambient black carbon (marker of traffic pollution) and Delta-C (marker of woodsmoke) concentrations estimated at each study subject’s residence using a land use regression model, increased but imprecise and not statistically significant rates of STEMI were found to be associated with increased concentrations of these source-specific PM constituents in the previous 2 and 3 days. However, an increased rate of STEMI associated with increased concentrations of these PM constituents was not found on the same day, as originally hypothesized.
Previously, in a similar case-crossover study, Gardner et al. (2014) reported an increased rate of STEMI associated with increased PM2.5 (measured at a fixed site in Rochester, NY) concentrations in the previous 1 h, suggesting that mechanistically, the hour before symptom onset for STEMI is an important time to estimate PM concentrations. A second analysis by Evans et al. (2017) examined whether increases in concentrations of source-specific PM constituents (black carbon and Delta-C) also triggered STEMI in the previous few hours. However, increased rates of STEMI associated with increased pollutant concentrations in the previous 1, 12, 24, 48, 72, or 96 h were found. In that analysis, the associations with pollutant concentrations were examined for the same lag times (previous few hours before symptom onset) as the Gardner et al. (2014) analysis, providing reasonable temporal alignment with the estimated symptom onset time. However, there was likely to be spatial misalignment (Peng and Bell 2010) since all subjects were assigned pollutant values from that fixed monitoring station, no matter how far they lived from that monitoring station and Wang et al. (2011b) had shown spatial inhomogeneity in the BC and Delta-C concentrations. This spatial misalignment and exposure error likely resulted in bias towards the null and underestimates of effect, perhaps obscuring any increased rates of STEMI associated with central site measured black carbon and Delta-C concentrations if they truly existed (Evans et al. 2017).
Weichenthal et al. (2017) reported a significant 6% increase in the odds of MI hospital admissions associated with increased concentrations of PM2.5 in the previous 3 days among elderly subjects (≥ 65 years of age), but not young subjects (< 65), among residents of three regions of British Columbia, Canada. Further, the strongest associations were observed in the cold season when monthly mean biomass burning contributions to PM2.5 (based on ambient levoglucosan/PM2.5) were in the highest tertile (OR = 1.19; 95% CI = 1.04, 1.36). Our Delta-C findings, although not statistically significant, are consistent with this study.
There has been limited work examining cardiovascular health effects of woodsmoke exposure (Naeher et al. 2007; Sigsgaard et al. 2015). Studies done in occupationally exposed firefighters and using controlled woodsmoke exposures have had mixed results, with some, but not all, reporting increased pulmonary and systemic inflammation and hemostatic responses (Barregard et al. 2006, 2008; Forchhammer et al. 2012; Hejl et al. 2013; Swiston et al. 2008; Tan et al. 2000). However, some studies have reported null findings for acute cardiovascular events in populations exposed to forest fire smoke (Delfino et al. 2009; Hanigan et al. 2008; Henderson et al. 2011; Johnston et al. 2007; Sigsgaard et al. 2015). In cities where woodsmoke PM is a large component of total winter PM, increased concentrations in the winter have been associated with increased cardiorespiratory admissions (Fairley 1999; Sanhueza et al. 2009; Schwartz et al. 1993). Other community-wide and in-home intervention studies have reported improved levels of inflammatory, endothelial function, blood pressure, and myocardial ischemia biomarkers, as well as reduced cardiorespiratory mortality associated with reduced woodsmoke exposures and concentrations (Allen et al. 2009; Johnston et al. 2013; McCracken et al. 2007, 2011). Previously, Croft et al. (2017) reported increased fibrinogen levels associated with increased Delta-C concentrations in the previous 12 h. Those findings are consistent with the studies showing cardiovascular responses to woodsmoke.
In this analysis, a land use regression model (Su et al. 2015) was used to estimate 24-h average Delta-C and black carbon concentrations at each subject’s residence providing greater spatial alignment than the previous analyses (Gardner et al. 2014; Evans et al. 2017). However, concentrations could only be estimated for the day of symptom onset, and not the specific hour(s), resulting in temporal misalignment. This temporal exposure error likely resulted in a bias towards the null and underestimates of effect. Further, the rate of STEMI associated with increased land use regression estimated black carbon and Delta-C concentrations in the previous few hours could not be estimated. Thus, to properly assess the rate of STEMI (and likely other acute cardiopulmonary events with a short triggering time) associated with short-term (< 24 h) increases in concentrations of source-specific PM constituents (e.g., black carbon and Delta-C), future studies should have a larger sample size (to increase statistical power) and use more sophisticated spatial-temporal models that can minimize both temporal and spatial misalignment. These models should be able to accurately estimate hourly pollutant concentrations at each subject’s residence. As investigators use epidemiology studies (e.g., panel, cohort, and case-control studies) to examine biologic mechanisms of air pollution-mediated acute cardiorespiratory events that may act on hourly time scales rather than days, weeks, and months, these more sophisticated spatial-temporal models will be needed.
Source of funding
This work was supported by grants from the New York State Energy Research and Development Authority (contract no. 32971) and National Institute of Environmental Health Sciences (grant no. P30 ES001247). Daniel Croft and Kristin Evans were supported by a National Institutes of Health training grant (T32-HL066988-1).
References
Allen RW, Leckie S, Millar G, Brauer M (2009) The impact of wood stove technology upgrades on indoor residential air quality. Atmos Environ 43:5908–5915
Barregard L, Sallsten G, Andersson L et al (2008) Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med 65(5):319–324
Barregard L, Sallsten G, Gustafson P et al (2006) Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol 18(11):845–853
Croft DP, Cameron SJ, Morrell CN, Lowenstein CJ, Ling F, Zareba W, Hopke PK, Utell MJ, Thurston SW, Thevenet-Morrison K, Evans KA, Chalupa D, Rich DQ (2017) Associations between ambient wood smoke and other particulate pollutants and biomarkers of systemic inflammation, coagulation, and thrombosis in cardiac patients. Environ Res 154:352–361
Delfino RJ, Brummel S, Wu J et al (2009) The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occup Environ Med 66(3):189–197
Evans KA, Hopke PK, Utell MJ, Kane C, Thurston SW, Ling FS, Chalupa D, Rich DQ (2017) Triggering of ST-elevation myocardial infarction by ambient wood smoke and other particulate and gaseous pollutants. J Expo Sci Environ Epidemiol 27:198–206
Fairley D (1999) Daily mortality and air pollution in Santa Clara County, California: 1989–1996. Environ Health Perspect 107(8):637–641
Forchhammer L, Moller P, Riddervold IS et al (2012) Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol 9:7
Gardner B, Ling F, Hopke PK, Frampton MW, Utell MJ, Zareba W, Cameron SJ, Chalupa D, Kane C, Kulandhaisamy S, Topf MC, Rich DQ (2014) Ambient fine particulate air pollution triggers ST-elevation myocardial infarction, but not non-ST elevation myocardial infarction: a case-crossover study. Part Fibre Toxicol 11:1
Hanigan IC, Johnston FH, Morgan GG (2008) Vegetation fire smoke, indigenous status and cardio-respiratory hospital admissions in Darwin, Australia, 1996–2005: a time-series study. Environ Health 7:42
Hejl AM, Adetona O, Diaz-Sanchez D et al (2013) Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. J Occup Environ Hyg 10(4):173–180
Henderson SB, Brauer M, MacNab YC, Kennedy SM (2011) Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environ Health Perspect 119:1266–1271
Johnston FH, Bailie RS, Pilotto LS, Hanigan IC (2007) Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health 7:240
Johnston FH, Hanigan IC, Henderson SB, Morgan GG (2013) Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: retrospective analysis of daily mortality, 1994–2007. BMJ 346:e8446
Larson TV, JG S, Baribeau AM, Buzzelli M, Setton EM, Brauer M (2007) A spatial model of urban winter woodsmoke concentrations. Environ Sci Technol 41:2429–2436
Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H (2001) Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology 12:186–192
McCracken J, Smith KR, Stone P, Diaz A, Arana B, Schwartz J (2011) Intervention to lower household wood smoke exposure in Guatemala reduces ST-segment depression on electrocardiograms. Environ Health Perspect 119(11):1562–1568
McCracken JP, Smith KR, Diaz A, Mittleman MA, Schwartz J (2007) Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect 115(7):996–1001
Mustafic H, Jabre P, Caussin C, Murad MH, Escolano S, Tafflet M, Périer MC, Marijon E, Vernerey D, Empana JP, Jouven X (2012) Main air pollutants and myocardial infarction: a systematic review and meta-analysis. JAMA 307:713–721
Naeher LP, Brauer M, Lipsett M et al (2007) Woodsmoke health effects: a review. Inhal Toxicol 19(1):67–106
Peng RD, Bell ML (2010) Spatial misalignment in time series studies of air pollution and health data. Biostatistics 11:720–740
Pope CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, Le V, Horne BD (2015) Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of ST-segment elevation acute coronary events. J Am Heart Assoc 4(12)
Sanhueza PA, Torreblanca MA, Diaz-Robles LA, Schiappacasse LN, Silva MP, Astete TD (2009) Particulate air pollution and health effects for cardiovascular and respiratory causes in Temuco, Chile: a wood-smoke-polluted urban area. J Air Waste Manag Assoc 59(12):1481–1488
Schwartz J, Slater D, Larson TV, Pierson WE, Koenig JQ (1993) Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am Rev Respir Dis Apr 147(4):826–831
Simoneit BRT (2002) Biomass burning—a review of organic tracers for smoke from incomplete combustion. Appl Geochem 17:129–162
Sigsgaard T, Forsberg B, Annesi-Maesano I, Blomberg A, Bølling A, Boman C, Bønløkke J, Brauer M, Bruce N, Héroux ME, Hirvonen MR, Kelly F, Künzli N, Lundbäck B, Moshammer H, Noonan C, Pagels J, Sallsten G, Sculier JP, Brunekreef B (2015) Health impacts of anthropogenic biomass burning in the developed world. Eur Respir J 46(6):1577–1588
Su JG, Larson TV, Baribeau AM, Brunekreef B, Rensing MD, Buzzelli M (2007) Spatial modeling for air pollution monitoring network design: example of residential woodsmoke. J Air Waste Manag Assoc 57:893–900
Su J, Hopke PK, Tian Y, Baldwin N, Thurston SW, Evans K, Rich DQ (2015) Modeling particulate matter concentrations measured through mobile monitoring in a machine learning approach. Atmos Environ 122:477–483
Suglia SF, Gryparis A, Schwartz J, Wright RJ (2008) Association between traffic-related black carbon exposure and lung function among urban women. Environ Health Perspect 116:1333–1337
Swiston JR, Davidson W, Attridge S, Li GT, Brauer M, van Eeden SF (2008) Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J 32(1):129–138
Tan WC, Qiu D, Liam BL et al (2000) The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med 161(4 Pt 1):1213–1217
Wang Y, Hopke PK, Rattigan OV, Xia X (2011a) Characterization of residential wood combustion particles using the two-wavelength aethalometer. Environ Sci Technol 45:7387–7393
Wang Y, Hopke PK, Utell MC (2011b) Urban-scale spatial-temporal variability of black carbon and winter residential wood combustion particles. Aerosol Air Qual Res 11:473–481
Wang Y, Hopke PK, Rattigan OV, Chalupa DC, Utell MJ (2012a) Multiple-year black carbon measurements and source apportionment using Delta-C in Rochester, New York. J Air Waste Manag Assoc 62:880–887
Wang Y, Hopke PK, Xia X, Rattigan OV, Chalupa DC, Utell MJ (2012b) Source apportionment of airborne particulate matter using inorganic and organic species as tracers. Atmos Environ 55:525–532
Weichenthal S, Kulka R, Lavigne E, van Rijswijk D, Brauer M, Villeneuve PJ, Steib D, Joseph L, Burnett RT (2017) Biomass burning as a source of ambient fine particulate air pollution and acute myocardial infarction. Epidemiology 28(3):329–337
Zhang Q, Qi W, Yao W, Wang M, Chen Y, Zhou Y (2016) Ambient particulate matter (PM/PM) exposure and emergency department visits for acute myocardial infarction in Chaoyang District, Beijing, China during 2014: a case-crossover study. J Epidemiol 26:538–545
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rich, D.Q., Utell, M.J., Croft, D.P. et al. Daily land use regression estimated woodsmoke and traffic pollution concentrations and the triggering of ST-elevation myocardial infarction: a case-crossover study. Air Qual Atmos Health 11, 239–244 (2018). https://doi.org/10.1007/s11869-017-0537-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11869-017-0537-1