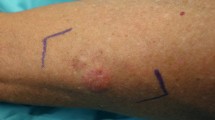
Opinion statement
Cutaneous sarcoma is a group of malignant mesenchymal tumors primarily involving the dermis, and it is characterized by extreme clinicopathological heterogeneity. Although its occurrence rate is rare, dermatofibrosarcoma protuberans (DFSP) is one of the most common types of dermal sarcoma. DFSP grows slowly and tends to relapse locally after inadequate resection. There are various histological variants of DFSP tumors and it often mimics benign lesions such as dermatofibroma and scar, which make accurate diagnosis difficult and delayed, and some cases progress to the stage where the tumor is unresectable. Recent advancements in cancer genetics and molecular biology methods have elucidated the COL1A1-PDGFB fusion gene, some novel fusion gene variants and pathways related to DFSP pathogenesis that have resulted in the evolution of cutaneous sarcoma diagnosis and treatment. For example, some clinical studies have confirmed the efficacy of imatinib methylate, an αPDGFR-targeted therapy for unresectable or metastatic DFSP. The present review summarizes recent updates in DFSP research, diagnostics, and treatment.



Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Rouhani P, Fletcher CD, Devesa SS, Toro JR. Cutaneous soft tissue sarcoma incidence patterns in the U.S. : an analysis of 12,114 cases. Cancer. 2008;113(3):616–27. https://doi.org/10.1002/cncr.23571.
Hoffmann EI. Über das knollentreibende Fibrosarkom der Haut (Dermatofibrosarkoma protuberans). Dermatology. 1925;43(1–2):1–28.
Llombart B, Serra-Guillen C, Monteagudo C, Lopez Guerrero JA, Sanmartin O. Dermatofibrosarcoma protuberans: a comprehensive review and update on diagnosis and management. Semin Diagn Pathol. 2013;30(1):13–28. https://doi.org/10.1053/j.semdp.2012.01.002.
Kim HJ, Lee JY, Kim SH, Seo YJ, Lee JH, Park JK, et al. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: comparison with factor XIIIa and CD34. Br J Dermatol. 2007;157(2):319–24. https://doi.org/10.1111/j.1365-2133.2007.08033.x.
Kahn HJ, Fekete E, From L. Tenascin differentiates dermatofibroma from dermatofibrosarcoma protuberans: comparison with CD34 and factor XIIIa. Hum Pathol. 2001;32(1):50–6. https://doi.org/10.1053/hupa.2001.21137.
West RB, Harvell J, Linn SC, Liu CL, Prapong W, Hernandez-Boussard T, et al. Apo D in soft tissue tumors: a novel marker for dermatofibrosarcoma protuberans. Am J Surg Pathol. 2004;28(8):1063–9.
Criscito MC, Martires KJ, Stein JA. Prognostic factors, treatment, and survival in dermatofibrosarcoma protuberans. JAMA Dermatol. 2016;152(12):1365–71. https://doi.org/10.1001/jamadermatol.2016.1886.
Brenner W, Schaefler K, Chhabra H, Postel A. Dermatofibrosarcoma protuberans metastatic to a regional lymph node. Report of a case and review. Cancer. 1975;36(5):1897–902.
Jha P, Moosavi C, Fanburg-Smith JC. Giant cell fibroblastoma: an update and addition of 86 new cases from the armed forces Institute of Pathology, in honor of Dr. Franz M Enzinger. Ann Diagn Pathol. 2007;11(2):81–8. https://doi.org/10.1016/j.anndiagpath.2006.12.010.
Zamecnik M, Michal M, Chlumska A. Composite dermatofibrosarcoma protuberans-giant cell fibroblastoma recurring as Bednar tumor-giant cell fibroblastoma with mucoid lakes and with amputation neuroma. Cesk Patol. 2002;38(4):173–7.
Bednar B. Storiform neurofibromas of the skin, pigmented and nonpigmented. Cancer. 1957;10(2):368–76.
Dupree WB, Langloss JM, Weiss SW. Pigmented dermatofibrosarcoma protuberans (Bednar tumor). A pathologic, ultrastructural, and immunohistochemical study. Am J Surg Pathol. 1985;9(9):630–9.
Suehara Y, Yazawa Y, Hitachi K. Metastatic Bednar tumor (pigmented dermatofibrosarcoma protuberans) with fibrosarcomatous change: a case report. J Orthop Sci. 2004;9(6):662–5. https://doi.org/10.1007/s00776-004-0831-2.
Kagoura M, Toyoda M, Nagahori H, Makino T, Morohashi M. An ultrastructural and immunohistochemical study of pigmented dermatofibrosarcoma protuberans (Bednar tumor). Eur J Dermatol. 1999;9(5):366–9.
Seo IS, Goheen M, Min KW. Bednar tumor: report of a case with immunohistochemical and ultrastructural study. Ultrastruct Pathol. 2003;27(3):205–10.
Bakry O, Attia A. Atrophic dermatofibrosarcoma protuberans. J Dermatol Case Rep. 2012;6(1):14–7. https://doi.org/10.3315/jdcr.2012.1089.
Lambert WC, Abramovits W, Gonzalez-Sevra A, Souchon E, Schwartz RA, Little WP Jr. Dermatofibrosarcoma non-protuberans: description and report of five cases of a morpheaform variant of dermatofibrosarcoma. J Surg Oncol. 1985;28(1):7–11.
Martin L, Combemale P, Dupin M, Chouvet B, Kanitakis J, Bouyssou-Gauthier ML, et al. The atrophic variant of dermatofibrosarcoma protuberans in childhood: a report of six cases. Brit J Dermatol. 1998;139(4):719–25.
Marini M, Saponaro A, Magarinos G, de Baldrich A, Lynch P, Remorino L. Congenital atrophic dermatofibrosarcoma protuberans. Int J Dermatol. 2001;40(7):448–50.
Sabater-Marco V, Perez-Valles A, Berzal-Cantalejo F, Rodriguez-Serna M, Martinez-Diaz F, Martorell-Cebollada M. Sclerosing dermatofibrosarcoma protuberans (DFSP): an unusual variant with focus on the histopathologic differential diagnosis. Int J Dermatol. 2006;45(1):59–62. https://doi.org/10.1111/j.1365-4632.2004.02340.x.
Hattori H. Nodular sclerotic change in dermatofibrosarcoma protuberans: a potential diagnostic problem. Br J Dermatol. 2003;148(2):357–60.
Diaz-Cascajo C, Weyers W, Borghi S. Sclerosing dermatofibrosarcoma protuberans. J Cutan Pathol. 1998;25(8):440–4.
Tran P, Henderson GP, McLemore M. An unusual clinical presentation of myxoid dermatofibrosarcoma protuberans with a prominent vasculature: a potential pitfall in the diagnosis of myxoid soft tissue tumors. J Cutan Pathol. 2018;45(6):419–22. https://doi.org/10.1111/cup.13130.
Sato N, Kimura K, Tomita Y. Recurrent dermatofibrosarcoma protuberans with myxoid and fibrosarcomatous changes paralleled by loss of CD34 expression. J Dermatol. 1995;22(9):665–72.
Zamecnik M, Michal M. Myxoid variant of dermatofibrosarcoma protuberans with fibrosarcomatous areas. Zentralbl Pathol. 1993;139(4–5):373–6.
Mentzel T, Scharer L, Kazakov DV, Michal M. Myxoid dermatofibrosarcoma protuberans: clinicopathologic, immunohistochemical, and molecular analysis of eight cases. Am J Dermatopathol. 2007;29(5):443–8. https://doi.org/10.1097/DAD.0b013e318145413c.
Calonje E, Fletcher CD. Myoid differentiation in dermatofibrosarcoma protuberans and its fibrosarcomatous variant: clinicopathologic analysis of 5 cases. J Cutan Pathol. 1996;23(1):30–6.
Al-Zaid T, Khoja H. Acral dermatofibrosarcoma protuberans with myoid differentiation: a report of 2 cases. J Cutan Pathol. 2017;44(9):794–7. https://doi.org/10.1111/cup.12982.
Kerob D, Porcher R, Verola O, Dalle S, Maubec E, Aubin F, et al. Imatinib mesylate as a preoperative therapy in dermatofibrosarcoma: results of a multicenter phase II study on 25 patients. Clin Cancer Res. 2010;16(12):3288–95. https://doi.org/10.1158/1078-0432.CCR-09-3401.
Sanz-Trelles A, Ayala-Carbonero A, Rodrigo-Fernandez I, Weil-Lara B. Leiomyomatous nodules and bundles of vascular origin in the fibrosarcomatous variant of dermatofibrosarcoma protuberans. J Cutan Pathol. 1998;25(1):44–9.
Banerjee SS, Harris M, Eyden BP, Hamid BN. Granular cell variant of dermatofibrosarcoma protuberans. Histopathology. 1990;17(4):375–8.
Wrotnowski U, Cooper PH, Shmookler BM. Fibrosarcomatous change in dermatofibrosarcoma protuberans. Am J Surg Pathol. 1988;12(4):287–93.
Llombart B, Monteagudo C, Sanmartin O, Lopez-Guerrero JA, Serra-Guillen C, Poveda A, et al. Dermatofibrosarcoma protuberans: a clinicopathological, immunohistochemical, genetic (COL1A1-PDGFB), and therapeutic study of low-grade versus high-grade (fibrosarcomatous) tumors. J Am Acad Dermatol. 2011;65(3):564–75. https://doi.org/10.1016/j.jaad.2010.06.020.
Lyu A, Wang Q. Dermatofibrosarcoma protuberans: a clinical analysis. Oncol Lett. 2018;16(2):1855–62. https://doi.org/10.3892/ol.2018.8802.
Hayakawa K, Matsumoto S, Ae K, Tanizawa T, Gokita T, Funauchi Y, et al. Risk factors for distant metastasis of dermatofibrosarcoma protuberans. J Orthop Traumatol. 2016;17(3):261–6. https://doi.org/10.1007/s10195-016-0415-x.
Sasaki M, Ishida T, Horiuchi H, MacHinami R. Dermatofibrosarcoma protuberans: an analysis of proliferative activity, DNA flow cytometry and p53 overexpression with emphasis on its progression. Pathol Int. 1999;49(9):799–806.
Takahira T, Oda Y, Tamiya S, Yamamoto H, Kawaguchi K, Kobayashi C, et al. Microsatellite instability and p53 mutation associated with tumor progression in dermatofibrosarcoma protuberans. Hum Pathol. 2004;35(2):240–5.
Hisaoka M, Okamoto S, Morimitsu Y, Tsuji S, Hashimoto H. Dermatofibrosarcoma protuberans with fibrosarcomatous areas. Molecular abnormalities of the p53 pathway in fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Virchows Arch. 1998;433(4):323–9.
Hiraki-Hotokebuchi Y, Yamada Y, Kohashi K, Yamamoto H, Endo M, Setsu N, et al. Alteration of PDGFRbeta-Akt-mTOR pathway signaling in fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Hum Pathol. 2017;67:60–8. https://doi.org/10.1016/j.humpath.2017.07.001.
Cleven AH, Al Sannaa GA, Briaire-de Bruijn I, Ingram DR, van de Rijn M, Rubin BP, et al. Loss of H3K27 tri-methylation is a diagnostic marker for malignant peripheral nerve sheath tumors and an indicator for an inferior survival. Mod Pathol. 2016;29(9):1113. https://doi.org/10.1038/modpathol.2016.103.
Pekmezci M, Cuevas-Ocampo AK, Perry A, Horvai AE. Significance of H3K27me3 loss in the diagnosis of malignant peripheral nerve sheath tumors. Mod Pathol. 2017;30(12):1710–9. https://doi.org/10.1038/modpathol.2017.97.
Shimizu A, O'Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, et al. The dermatofibrosarcoma protuberans-associated collagen type I alpha 1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res. 1999;59(15):3719–23.
McArthur G. Molecularly targeted treatment for dermatofibrosarcoma protuberans. Semin Oncol. 2004;31(2):30–6. https://doi.org/10.1053/j.seminoncol.2004.03.038.
• Nakamura I, Kariya Y, Okada E, Yasuda M, Matori S, Ishikawa O, et al. A novel chromosomal translocation associated with COL1A2-PDGFB gene fusion in dermatofibrosarcoma protuberans: PDGF expression as a new diagnostic tool. JAMA Dermatol. 2015;151(12):1330–7. https://doi.org/10.1001/jamadermatol.2015.2389. Reporting the novel fusion gene of DFSP; COL1A2-PDGFB.
• Dadone-Montaudie B, Alberti L, Duc A, Delespaul L, Lesluyes T, Perot G, et al. Alternative PDGFD rearrangements in dermatofibrosarcomas protuberans without PDGFB fusions. Mod Pathol. 2018;31(11):1683–93. https://doi.org/10.1038/s41379-018-0089-4. Next generation sequencing study reporting the novel fusion gene of DFSP; EMILIN2-PDGFD.
• Dickson BC, Hornick JL, CDM F, Demicco EG, Howarth DJ, Swanson D, et al. Dermatofibrosarcoma protuberans with a novel COL6A3-PDGFD fusion gene and apparent predilection for breast. Genes Chromosom Cancer. 2018;57(9):437–45. https://doi.org/10.1002/gcc.22663. Next generation sequencing study reporting the novel fusion gene of DFSP; COL6A3-PDGFD.
Eilers G, Czaplinski JT, Mayeda M, Bahri N, Tao D, Zhu M, et al. CDKN2A/p16 loss implicates CDK4 as a therapeutic target in imatinib-resistant dermatofibrosarcoma protuberans. Mol Cancer Ther. 2015;14(6):1346–53. https://doi.org/10.1158/1535-7163.MCT-14-0793.
Saab J, Rosenthal IM, Wang L, Busam KJ, Nehal KS, Dickson MA, et al. Dermatofibrosarcoma protuberans-like tumor with COL1A1 copy number gain in the absence of t(17;22). Am J Dermatopathol. 2017;39(4):304–9. https://doi.org/10.1097/DAD.0000000000000746.
Veronese F, Boggio P, Tiberio R, Gattoni M, Fava P, Caliendo V, et al. Wide local excision vs. Mohs Tubingen technique in the treatment of dermatofibrosarcoma protuberans: a two-centre retrospective study and literature review. J Eur Acad Dermatol Venereol. 2017;31(12):2069–76. https://doi.org/10.1111/jdv.14378.
Mendenhall WM, Zlotecki RA, Scarborough MT. Dermatofibrosarcoma protuberans. Cancer. 2004;101(11):2503–8. https://doi.org/10.1002/cncr.20678.
Acosta AE, Velez CS. Dermatofibrosarcoma Protuberans. Curr Treat Options in Oncol. 2017;18(9):56. https://doi.org/10.1007/s11864-017-0498-5.
Ng A, Nishikawa H, Lander A, Grundy R. Chemosensitivity in pediatric dermatofibrosarcoma protuberans. J Pediatr Hematol Oncol. 2005;27(2):100–2.
Suit H, Spiro I, Mankin HJ, Efird J, Rosenberg AE. Radiation in management of patients with dermatofibrosarcoma protuberans. J Clin Oncol. 1996;14(8):2365–9. https://doi.org/10.1200/JCO.1996.14.8.2365.
Sun LM, Wang CJ, Huang CC, Leung SW, Chen HC, Fang FM, et al. Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol. 2000;57(2):175–81.
Dagan R, Morris CG, Zlotecki RA, Scarborough MT, Mendenhall WM. Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol. 2005;28(6):537–9.
Chen YT, Tu WT, Lee WR, Huang YC. The efficacy of adjuvant radiotherapy in dermatofibrosarcoma protuberans: a systemic review and meta-analysis. J Eur Acad Dermatol Venereol. 2016;30(7):1107–14. https://doi.org/10.1111/jdv.13601.
Lemm D, Mugge LO, Mentzel T, Hoffken K. Current treatment options in dermatofibrosarcoma protuberans. J Cancer Res Clin Oncol. 2009;135(5):653–65. https://doi.org/10.1007/s00432-009-0550-3.
Shimizu A, O'Brien KP, Sjoblom T, Pietras K, Buchdunger E, Collins VP, et al. The dermatofibrosarcoma protuberans-associated collagen type Ialpha1/platelet-derived growth factor (PDGF) B-chain fusion gene generates a transforming protein that is processed to functional PDGF-BB. Cancer Res. 1999;59(15):3719–23.
Ugurel S, Mentzel T, Utikal J, Helmbold P, Mohr P, Pfohler C, et al. Neoadjuvant imatinib in advanced primary or locally recurrent dermatofibrosarcoma protuberans: a multicenter phase II DeCOG trial with long-term follow-up. Clin Cancer Res. 2014;20(2):499–510. https://doi.org/10.1158/1078-0432.CCR-13-1411.
McArthur GA, Demetri GD, van Oosterom A, Heinrich MC, Debiec-Rychter M, Corless CL, et al. Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: imatinib target exploration consortium study B2225. J Clin Oncol. 2005;23(4):866–73. https://doi.org/10.1200/JCO.2005.07.088.
Stacchiotti S, Pedeutour F, Negri T, Conca E, Marrari A, Palassini E, et al. Dermatofibrosarcoma protuberans-derived fibrosarcoma: clinical history, biological profile and sensitivity to imatinib. Int J Cancer. 2011;129(7):1761–72. https://doi.org/10.1002/ijc.25826.
Oh E, Jeong HM, Kwon MJ, Ha SY, Park HK, Song JY, et al. Unforeseen clonal evolution of tumor cell population in recurrent and metastatic dermatofibrosarcoma protuberans. PLoS One. 2017;12(10):e0185826. https://doi.org/10.1371/journal.pone.0185826.
Kitagawa D, Yokota K, Gouda M, Narumi Y, Ohmoto H, Nishiwaki E, et al. Activity-based kinase profiling of approved tyrosine kinase inhibitors. Genes Cells. 2013;18(2):110–22. https://doi.org/10.1111/gtc.12022.
Fu Y, Kang H, Zhao H, Hu J, Zhang H, Li X, et al. Sunitinib for patients with locally advanced or distantly metastatic dermatofibrosarcoma protuberans but resistant to imatinib. Int J Clin Exp Med. 2015;8(5):8288–94.
Xiao W, Que Y, Peng R, Ding Y, Zhao J, Wen X, et al. A favorable outcome of advanced dermatofibrosarcoma protuberans under treatment with sunitinib after imatinib failure. Onco Targets Ther. 2018;11:2439–43. https://doi.org/10.2147/OTT.S150235.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–109. https://doi.org/10.1158/0008-5472.CAN-04-1443.
Kamar FG, Kairouz VF, Sabri AN. Dermatofibrosarcoma protuberans (DFSP) successfully treated with sorafenib: case report. Clin Sarcoma Res. 2013;3(1):5. https://doi.org/10.1186/2045-3329-3-5.
Miyagawa T, Kadono T, Kimura T, Saigusa R, Yoshizaki A, Miyagaki T, et al. Pazopanib induced a partial response in a patient with metastatic fibrosarcomatous dermatofibrosarcoma protuberans without genetic translocations resistant to mesna, doxorubicin, ifosfamide and dacarbazine chemotherapy and gemcitabine-docetaxel chemotherapy. J Dermatol. 2017;44(3):e21–e2. https://doi.org/10.1111/1346-8138.13717.
Tsuchihashi K, Kusaba H, Yamada Y, Okumura Y, Shimokawa H, Komoda M, et al. Programmed death-ligand 1 expression is associated with fibrosarcomatous transformation of dermatofibrosarcoma protuberans. Mol Clin Oncol. 2017;6(5):665–8. https://doi.org/10.3892/mco.2017.1197.
Osio A, Xu S, El Bouchtaoui M, Leboeuf C, Gapihan G, Lemaignan C, et al. EGFR is involved in dermatofibrosarcoma protuberans progression to high grade sarcoma. Oncotarget. 2018;9(9):8478–88. https://doi.org/10.18632/oncotarget.23899.
Bigby SM, Oei P, Lambie NK, Symmans PJ. Dermatofibrosarcoma protuberans: report of a case with a variant ring chromosome and metastases following pregnancy. J Cutan Pathol. 2006;33(5):383–8. https://doi.org/10.1111/j.0303-6987.2006.00404.x.
Meng T, Shi XH, Wu SF, Luo YF, Wang XJ, Long X. Hormone receptors analysis in Chinese patients with dermatofibrosarcoma protuberans. J Surg Oncol. 2018;118:157–66. https://doi.org/10.1002/jso.25117.
Kreicher KL, Honda KS, Kurlander DE, Bordeaux JS. Hormone receptor expression in patients with dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2016;75(6):1205–9. https://doi.org/10.1016/j.jaad.2016.07.011.
Stacchiotti S, Astolfi A, Gronchi A, Fontana A, Pantaleo MA, Negri T, et al. Evolution of dermatofibrosarcoma protuberans to DFSP-derived fibrosarcoma: an event marked by epithelial-mesenchymal transition-like process and 22q loss. Mol Cancer Res. 2016;14(9):820–9. https://doi.org/10.1158/1541-7786.MCR-16-0068.
Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011;17(9):2613–8. https://doi.org/10.1158/1078-0432.CCR-10-2156.
Holm K, Grabau D, Lovgren K, Aradottir S, Gruvberger-Saal S, Howlin J, et al. Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Mol Oncol. 2012;6(5):494–506. https://doi.org/10.1016/j.molonc.2012.06.002.
Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, et al. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma and advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol. 2018;19(5):649–59. https://doi.org/10.1016/S1470-2045(18)30145-1.
Kim J, Lee Y, Lu X, Song B, Fong KW, Cao Q, et al. Polycomb- and methylation-independent roles of EZH2 as a transcription activator. Cell Rep. 2018;25(10):2808–20 e4. https://doi.org/10.1016/j.celrep.2018.11.035.
Dickson MA, Mahoney MR, Tap WD, D'Angelo SP, Keohan ML, Van Tine BA, et al. Phase II study of MLN8237 (Alisertib) in advanced/metastatic sarcoma. Ann Oncol. 2016;27(10):1855–60. https://doi.org/10.1093/annonc/mdw281.
Kanamori S, Kajihara I, Kanazawa-Yamada S, Otsuka-Maeda S, Ihn H. Expression of aurora kinase A expression in dermatofibrosarcoma protuberans. J Dermatol. 2018;45(4):507–8. https://doi.org/10.1111/1346-8138.14235.
Acknowledgements
We are very grateful to Dr. Y. Yamada, K. Kohashi, and I. Kinoshita at Kyushu University Hospital for helpful discussions. We also thank Dr. Y. Ohshiro at Matsuyama Red Cross Hospital for kindly providing the clinical samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skin Cancer
Rights and permissions
About this article
Cite this article
Iwasaki, T., Yamamoto, H. & Oda, Y. Current Update on the Molecular Biology of Cutaneous Sarcoma: Dermatofibrosarcoma Protuberans. Curr. Treat. Options in Oncol. 20, 29 (2019). https://doi.org/10.1007/s11864-019-0628-3
Published:
DOI: https://doi.org/10.1007/s11864-019-0628-3