Abstract
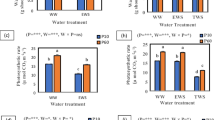
The objective of the present investigation was to evaluate Al tolerance in three Vigna species viz. V. radiata (‘Pusa-672’), V. mungo (‘Mash-114’) and V. umbellata (‘RBL-6’) under Al stress conditions. All three Vigna species were assessed in hydroponic assay in various concentration of Al (0, 74 and 185 μM) for 48 h. Variations in the Al tolerance were analysed based on various traits such as root elongation rate, re-growth after hematoxylin staining, accumulation of aluminium and callose and their localization, H2O2, lipid peroxidation and antioxidant enzymes activity. Aluminium stress caused inhibition in root elongation rate and root re-growth and increased accumulation of aluminium, callose, H2O2 and lipid peroxidation in all three Vigna species. However, accumulation of aluminium, callose, H2O2 and lipid peroxidation was more in V. radiata (‘Pusa-672’) than in V. mungo (‘Mash-114’) and V. umbellata (‘RBL-6’). Higher activity of superoxide dismutase (SOD; EC 1.15.1.1), guaiacol peroxidase (GPX; EC 1.11.1.7) and ascorbate peroxidase (APX; EC 1.11.1.11) was observed in V. umbellata than in V. mungo and V. radiata. Transverse sections of roots were examined to confirm the localization of Al in the apoplastic or symplastic regions using fluorescent microscopy. In V. umbellata (‘RBL-6’) and V. mungo (‘Mash-114’), most of the Al was localised in the epidermal and cortical tissues indicating restricted movement of Al to the upper layers. In V. radiata, (‘Pusa-672’) more Al was localised in epidermal, cortical, and even endodermal tissues, suggesting its inability to restrict the Al in upper layers. Our findings suggest that V. umbellata as a potential genetic resource for Al tolerance and this trait can be introgressed through breeding programme to develop Al-tolerant genotypes in V. mungo and V. radiata.






Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- CAT:
-
Catalase
- GPX:
-
Guaiacol peroxidase
- H2O2 :
-
Hydrogen Peroxide
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- SOD:
-
superoxide dismutase
- TBARS:
-
Thiobarbituric acid reacting substances
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ahn SJ, Matsumoto H (2006) The role of the plasma membrane in the response of plants roots to aluminum toxicity. Plant Signal Behav 1:37–45
Alia Prasad KV, Pardha SK, Saradhi P (1995) Effect of zinc on free radical and proline in Brassica and Cajanus. Phytochemistry 39:45–47
Alvim MN, Ramos FT, Oliveira DC, Isaias RMS, França MGC (2012) Aluminium localization and toxicity symptoms related to root growth inhibition in rice (Oryza sativa L.) seedlings. J Biosci 37:1079–1088
Andrade LRM, Ikeda M, Ishizuka J (1997) Stimulation of organic acid excretion by roots of aluminum-tolerant and aluminium sensitive wheat varieties under aluminium stress. R Bras Fisiol Veg 9:27–34
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55:373–399
Basu U, Good AG, Taylor GJ (2001) Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant, Cell Environ 24:1269–1278
Boscolo PRS, Menossi M, Jorge RA (2003) Al-induced oxidative stress in maize. Phytochemistry 62:181–189
Cakmak I, Horst WJ (1991) Effect of Al on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tips of soybean (Glycine max L.). Physiol Plant 83:463–468
Castillo FJ, Penel C, Greppin H (1984) Peroxidase release induced by ozone in Sedum album leaves. Plant Physiol 74:846–851
Choudhary AK, Singh D, Kumar J (2011) A comparative study of screening methods for tolerance to aluminum toxicity in pigeonpea [Cajanus cajan (L.) Millspaugh]. Aust J Crop Sci 5:1419–1426
Darko E, Ambrus H, Banyai ES, Fodor J, Bakos F, Barnabas B (2004) Aluminum toxicity, Al-tolerance and oxidative stress in an aluminum sensitive wheat genotype and in Al-tolerant lines developed by in vitro microspore selection. Plant Sci 166:583–591
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Dhindsa RS, Plump-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Dipierro N, Mondelli D, Paciolla C, Brunetti G, Dipierro S (2005) Changes in the ascorbate system in the response of pumpkin Cucurbita pepo L.) roots to aluminium stress. J Plant Physiol 162:529–536
Du B, Nian H, Zhang Z, Yang C (2010) Effects of aluminum on superoxide dismutase and peroxidase activities, and lipid peroxidation in the roots and calluses of soybeans differing in aluminium tolerance. Acta Physiol Plant 32:883–890
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminium stress and/or oxidative stress. Plant Physiol 122:657–665
Farooq MA, Ali S, Hameed A, Ishaque W, Mahmood K, Iqbal Z (2013) Alleviation of cadmium toxicity by silicon is related to elevated photosynthesis, antioxidant enzymes, suppressed cadmium uptake and oxidative stress in cotton. Ecotoxicol Environ Saf 96:242–249
Foy CD (1988) Plant adaptation to acid, aluminium-toxic soils. Commun Soil Sci Plant Anal 19:959–987
Giannakoula A, Moustakas M, Syros T, Yupsanis T (2010) Aluminium stress induces up-regulation of an efficient antioxidant system in the Al-tolerant maize line but not in the Al-sensitive line. Environ Exp Bot 67:487–494
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier.-. Funct Plant Biol 32:481–494
Guevara P, Poschenrieder Ch, Barceló (1992) Differential response of four maize (Zea mays L.) varieties to aluminum toxicity. Sueloy Planta 1:714–721
Haug A, Caldwell CR (1985) Aluminium toxicity in plants: the role of root plasma membrane and calmodulin. In: John JB St, Berlin E, Jackson E, Otawa PC (Eds.) Frontiers of membrane Research in Agriculture, (Rowman and Allanheld), pp 359–381
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives in Bioch Biophy 125:189–198
Horst WJ, Asher CJ, Cakmak I, Szulkiewica P, Wissemeier AH (1992) Short-term responses of soybean roots to aluminum. J Plant Physiol 140:174–178
Kauss H (1992) Callose and callose synthase. In: SJ Gurr, MJ McPherson, DJ Bowles (eds) Molecular plant morphology: a practical approach. vol 2, Oxford University Press, New York, pp 1–8
Kochian LV (1995) Cellular mechanism of aluminium toxicity and resistance in plants. Ann Rev Plant Physiol Plant Mol Biol 46:237–260
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Ann Rev Plant Biol 55:459–493
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Kuo MC, Kao CH (2003) Aluminum effects on lipid peroxidation and antioxidative enzyme activities in rice leaves. Biol Plant 46:149–152
Lee SH, Ahsan N, Lee KW, Kim DH, Lee DG, Kwak SS, Kwon SY, Kim TH, Lee BH (2007) Simultaneous overexpression of both CuZn superoxide dismutase and ascorbate peroxidase in transgenic tall fescue plants confers increased tolerance to a wide range of abiotic stresses. J Plant Physiol 164:1626–1638
Ma BH, Wan JM, Shen ZG (2007) H2O2 production and antioxidant responses in seeds and early seedling of two different rice varieties exposed to aluminum. Plant Growth Regul 52:91–100
Mishra S, Srivastava S, Tripathi RD, Govindarajan R, Kuriakose SV, Prasad MNV (2006) Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem 44:25–37
Mittler R, Vanderauwera S, Gallery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Oteiza PI (1994) A mechanism for the stimulatory effect of aluminum on iron-induced lipid peroxidation. Arch Biochem Biophys 308:374–379
Parker DR (1995) Root growth analysis: an underutilised approach to understanding aluminium rhizotoxicity. Plant Soil 171:151–157
Polle E, Konzak CF, Kittrick JA (1978) Visual detection of aluminium tolerance levels in wheat by hematoxylin staining of seedlings roots. Crop Sci 18:823–827
Poschenrieder C, Gunse B, Corrales I, Barcelo J (2008) A glance into aluminium toxicity and resistance in plants. Sci Total Environ 400:356–368
Rao MV, Paliyath G, Ormrod DP (1996) Ultraviolet-B and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol 110:125–136
Ryan PR, DiTomaso JM, Kochian LV (1993) Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44:437–446
Scott BJ, Fisher JA, Spohr LJ (1992) Tolerance of australian wheat varieties to aluminium toxicity. Commun Soil Sci Plant Anal 23:509–526
Sharma P, Dubey RS (2007) Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep 26:2027–2038
Simon L, Smalley TJ, Jones JB Jr, Lasseigne FT (1994) Aluminum toxicity in tomato: part I. Growth and mineral nutrition. J Plant Nutr 17:293–306
Singh D, Chauhan SK (2011) Organic acids of crop plants in aluminium detoxification. Curr Sci 100:1509–1515
Singh D, Dikshit HK (2012) Comparison of aluminium tolerance in mung bean (Vigna radiata L.) and related species under low pH conditions. Paper presented in International Symposium on “Plant Soil Interaction at Low pH at University of Agricultural Sciences, Bangalore, Karnataka, India from October 18–22, pp 146–147
Singh D, Dikshit HK, Singh R (2012) Variation of aluminium tolerance in lentil. Plant Breed 131:751–761
Singh D, Dikshit HK, Kumar R (2015) Aluminium tolerance in lentil with monogenic inheritance pattern. Plant Breed 134:105–110
Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K (2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307:167–178
Tice KR, Parker DR, Demason DA (1992) Operationally defined apoplastic and symplastic aluminum fractions in root tips of aluminum-intoxicated wheat. Plant Physiol 100:309–318
Vázquez MD, Poschenrieder C, Corrales I, Barcelo J (1999) Change in apoplastic aluminium during the initial growth response to aluminium by roots of a tolerant maize variety. Plant Physiol 119:435–444
Wissemeier AH, Klotz F, Horst WJ (1987) Aluminium induced callose synthesis in roots of soybean (Glycine max L.). J Plant Physiol 129:487–492
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yin L, Junichi M, Wang S, Tsuji W, Tanaka K (2010) The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol 152:1406–1417
Zhang G, Hoddinott J, Taylor GJ (1994) Characterization of 1,3-β-D-glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J Plant Physiol 144:229–234
Zhen Y, Miao L, Su J, Liu SH, Yin YL, Wang SS, Pang YJ, Shen HG, Tian D, Qi JL, Yang YH (2009) Differential responses of antioxidative enzymes to aluminum stress in tolerant and sensitive soybean genotypes. J Plant Nutr 32:1255–1270
Acknowledgments
The authors thank to Head, Division of Genetics and Plant Physiology, Director and Joint Director Research, and Incharge National Phytotron Facility, Indian Agricultural Research Institute, New Delhi, India for their encouragement and providing research facilities to carry out this research work. This work was supported by the Indian Agricultural Research Institute, New Delhi, India (Project No. Gen09/16).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zwiazek.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, D., Pal, M., Singh, R. et al. Physiological and biochemical characteristics of Vigna species for Al stress tolerance. Acta Physiol Plant 37, 87 (2015). https://doi.org/10.1007/s11738-015-1834-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-015-1834-7