Abstract
Although important, phenological studies comparing congeneric species or the same species growing in different habitats are still scarce for the tropics. Herein, we integrate phylogeny, ecology and biometeorology to verify whether the phenophases of congeneric species Myrcia laruotteana and Myrcia amazonica or Clethra scabra differ when their populations inhabit wetland and drained habitats and to determine what abiotic factors affect the vegetative and reproductive phenophases of these species in distinct habitat patches. We collected data on phenological events of 80 trees for 1 year in Itacolomi State Park, Brazil, and related them to abiotic local factors. Contrary to our expectation, the phenophases of the congeneric species did not differ between habitats, but the reproductive phenophases of C. scabra did and was greater in drained soil. Phenophases of C. scabra were affected by the depth of the water table and maximum temperature in the wetland soil. Insolation, precipitation, maximum temperature and relative humidity influenced Myrcia and Clethra in the drained soil. The differences between C. scabra populations suggest that this species is phenotypically plastic and can present distinct phenophases depending on the habitat it inhabits. On the other hand, the congeneric Myrcia species may have similar phenophases in distinct habitats because of their shared similarities during their evolution. This study provides a better understanding of the ecology of these species and their adaptations to different abiotic conditions. Data of this nature are important in a changing world and can inform strategies for adaptive management.
Similar content being viewed by others
Introduction
Phenology, the timing of recurrent seasonal biological events, affects almost all aspects of ecology and evolution (Forrest and Miller-Rushing 2010). Phenophases (also known as phenological events) are observable stages or phases in the annual life cycle of a plant or animal (USA National Phenology Network 2019). Plant phenophases such as leaf budding, flowering and fruit ripening have been recorded for decades and are important to verify temporal changes in the species development (Gordo and Sanz 2009) and understand how plant communities are assembled (Wolkovich et al. 2013).
Various factors such as temperature, precipitation and solar radiation have been listed as being influential on plant phenophases over time (Bradley et al. 2011; Shen et al. 2011). Temperature, in particular, has been linked to the phenology of plants and insects, and studies have linked changes in the timing of some phenophases of these organisms with global warming (Bartomeus et al. 2011).
Another relevant but little explored issue in the search for the drivers of phenology is the shared evolutionary history of the species, i.e. the phylogeny (Davies et al. 2013). More recently, some studies have compared the phenology of related species inhabiting different habitats, and valuable information that helps to better understand the ecology and evolution of the species has been acquired (Alberto et al. 2013; Gugger et al. 2015). For example, phenological responses to climate change are often shared among closely related plant species, meaning that related species respond similarly to climate change (Davis et al. 2010).
Although some information is known, there is still a lack of studies linking phenophases of related species in different habitats to environmental factors, especially in the tropics. In the Atlantic Forest, for example, the frequency, average date and intensity of phenophases of related species vary according to soil type (Cambisol Vs Gleysol) (Cardoso et al. 2012). In other communities, however, the variation in flowering time is explained by abiotic variables, without a significant phylogenetic effect (Cortés-Flores et al. 2017). Further studies comparing related species in distinct environments may help to clarify these issues. In doing so, it is important to integrate disciplines such as biometeorology, ecology and evolutionary biology because multiple factors can interact and affect phenology in tropical regions (Wolkovich et al. 2014).
Here we compare congeneric species (i.e., species of the same genera) and a same species inhabiting nearby habitat patches with different soil water conditions (wetland and drained soils). The objectives of the study were to verify whether the phenophases of the congeneric Myrcia laruotteana and Myrcia amazonica species and Clethra scabra species differ depending on the habitat occupied as a function of environment, which abiotic factor(s) affects the phenophases of the populations in wetland and drained habitats and the nature of any effect. Our predictions were that (1) the phenophases of congeneric species should differ because although they share similarities in their life history, each species is adapted to a specific habitat; (2) phenophases of the same species should not differ because they have the same evolutionary history; and (3) that factors affecting plant phenophases should differ depending on the habitat occupied.
Materials and methods
Study areas
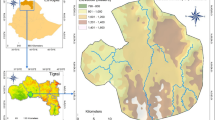
We performed this study in Itacolomi State Park (PEIT) in southeast Minas Gerais state, Brazil, whose area occupies part of the municipalities of Ouro Preto and Mariana (Fig. 1a). The park, created in 1967, occupies 7,543 ha in the southern part of the Serra do Espinhaço Biosphere Reserve (IEF 2019). The tropical climate has two well-defined seasons: a rainy, warm season (from October to March) and a dry, cold season (from April to September) (Climate-Data.org2019).
Aerial view of the wetland and drained study areas in Itacolomi State Park, Minas Gerais state, southeast Brazil (a) selected for characterizing the phenology of three arboreal species. M. laruotteana occurs only in the wetland area (b) and M. amazonica in the drained area. C. scabra occurs in both areas
Itacolomi Park is composed of a transition zone between the Atlantic Forest and Cerrado biomes, both of which are considered priority ecosystems for conservation (biodiversity hotspots) (Myers et al. 2000). Vegetation is dense and taller in the valleys, drainages and lower slopes because of the environmental and topographic heterogeneity than the sparse, shorter vegetation in the higher areas that are topped with rock fields (Fujaco et al. 2010).
We selected two adjacent areas to sample within a fragment of montane forest of about 100 ha and an average altitude of 1,350 m above sea level (Pedreira and de Sousa 2011). These areas are located near the Manso stream (20 °25′37″ S; 43 °30′18″ W). The area to the left of the stream, classified as wetland forest, is flat, and the soil is constantly flooded (Fig. 1b). Wetland forests are characterized by the presence of permanently soaked hydromorphic soils with surface water flowing into small channels with a certain drainage (Rodrigues 2004). The area to the right of the stream (in the direction of the water flow—North), classified as montane forest, had a sloping relief and drained soil (Fig. 1b) (Pedreira and de Sousa 2011). Montane forests are characterized by the presence of well-drained soils such as Nitosols, Cambisols or Latosols (Higuchi et al. 2008).
Study species
The target species of this study are the two congenerics, Myrcia laruotteana Cambes and Myrcia amazonica D.C., and Clethra scabra Pers. M. laruotteana commonly occurs in semi-deciduous seasonal forests, especially in wetland places and at the edges of rivers and streams (Sobral et al. 2013). At our study sites, it only occurs in the wetland area (Fig. 1b). M. amazonica, an evergreen tree typical of riparian zones of the Cerrado, semi-deciduous seasonal forests, and rock fields (Sobral et al. 2013), only grows in the drained area (Fig. 1b). C. scabra is also an arboreal species occurring in the interior of secondary forests with poor, moist, compact soils, and shallow inclination (Lorenzi 1998). It inhabits both study areas (wetland and drained soils).
Experimental design
We set up four 10 m transects each parallel to the Manso stream in the study areas (wetland and drained). Then, we identified and marked all individual trees (with DBH ≥ 5 cm) of the target species with numbered tags within a perimeter of 5 m on each side of the transects. We then randomly selected 20 individuals of each species from the set of marked trees in each area by picking 80 slips of papers from a container with numbered slips for all individuals. Thus, each study area had 40 individuals marked and selected for the phenology study: 20 M. laruotteana trees and 20 C. scabra trees in the wetland habitat, 20 M. amazonica trees and 20 C. scabra trees in the drained habitat.
Data collection
We visited the study areas every 15 days from November 2013 to October 2014 to estimate the vegetative and reproductive phenophases of the populations. Because the data was collected consistent throughout the study, this frequency is appropriate for estimating phenophases (Miller-Rushing et al. 2008), and the progression of all phenophases can be observed. During each visit, we observed the entire crown of the target trees using binoculars and registered the presence or absence of leaf budding, young leaves, mature leaves, leaf fall, flower budding, flowering and fruiting.
A piezometer was installed near each tree (up to 2 m) to measure the depth of the water table. The piezometers were made of 10 cm diameter plastic pipes with perforations throughout their length and installed 100 cm below the soil surface (Walthall and Lngram 1984). The depth of the water table was measured using a graduated tape during the bimonthly visits in the study areas.
We also obtained data for monthly precipitation (mm), maximum and minimum temperatures (°C), relative humidity (%), and insolation (hours) to relate to the phenophases for the study period from a meteorological station approximately 3.5 km from the study areas (the station belongs to Novelis do Brasil Ltda). We calculated the region’s average precipitation and temperature from 2004 to 2013, and those during the study year did not deviate significantly (precipitation: t = 1.32, p = 0.20; temperature: t = 0.44, p = 0.66).
Data analyses
An activity index (or percentage of individuals) was calculated for each phenophase in each month using the presence and absence data to estimate synchrony and determine the proportion of individuals sampled that are manifesting a certain phenophase in relation to the total individuals of the population (Bencke and Morellato 2002). The indexes were graphically represented for better interpretation.
We transformed the phenological data (the activity index, in percentage) into logit following the recommendation of Warton and Hui (2011) for transforming proportion data. The assumption of multivariate normality was checked through Mardia tests and homogeneity of variance–covariance matrices through Box’s M tests of equality of covariance (Hammer 2019). Data were normal (Mardia test (Myrcia): df = 84, Statistic = 0.05, p = 0.95; (Clethra): df = 84, Statistic = 0.20, p = 0.83) and variance–covariance matrices were homogeneous (Box’s M test (Myrcia): F21, 1780.2 = 1.83, p = 0.22; (Clethra): F21, 1780.2 = 1.63, p = 0.05). Spatial autocorrelation was also checked because the populations of both habitats were relatively close to each other in space using the autocorrelation function (ACF) and partial autocorrelation function (PACF) (Davis 1986) in the SPSS 23.0 program (IBM, Armonk, NY, USA). The results indicated that spatial autocorrelation was not a problem for Myrcia and Clethra populations.
We subjected the phenological data to a multivariate analysis of variance with repeated measures (repeated measures MANOVA) to verify differences in vegetative and reproductive phenophases of the congeneric Myrcia species and C. scabra species when inhabiting different habitat patches (study objective 1). MANOVA is used to assess the statistical significance of the effect of one or more independent variables on a set of two or more dependent variables (Weinfurt 1995). In this case, a repeated-measures approach was used because phenological data were measured repeatedly over time for the same individuals. The phenophases were used as measures of the populations in the different habitats (dependent variables: congeneric Myrcia species and C. scabra species in wetland and drained habitats). The Bonferroni correction was used for pairwise comparisons (Hammer 2019).
Multivariate multiple regression (MMR) analyses were performed to verify what (and how) abiotic factors influenced the phenophases of the populations inhabiting wetland and drained habitats in that period (study objective 2). Multiple responses (dependent variables) can be modeled using a set of predictor (independent) variables (Ford 2017). Phenophases were the dependent variables, and the abiotic factors were the independent variables (water table depth, precipitation, average maximum temperature, average minimum temperature, relative humidity, and insolation). MMR analyses were performed separately by species in each environment to verify which factors were related to the phenophases. The assumptions of linearity (if the residuals were normally distributed and homoscedastic) and multicollinearity (though variance inflation factor VIF < 10) were checked before the MMR analysis (Hammer 2019). SPSS 23.0 (IBM, Armonk, NY, USA) and PAST 3.26 (Hammer et al. 2001) software were used for the statistical tests.
Results
We verified no significant differences in the vegetative and reproductive phenophases of Myrcia inhabiting wetland and drained habitats (study objective 1, MANOVA test: F5, 7 = 1.88, p = 0.25, Wilks’ Λ = 0.27). M. laruotteana and M. amazonica remained evergreen over the year, and flower buds and flowers were produced at the end of the dry season (August and September) (Fig. 2). Fruits were produced between September and January (Fig. 2).
On the other hand, some of the phenophases of the two C. scabra populations differed (study objective 1, MANOVA test: F5, 7 = 76.23, p < 0.001, Wilks’ Λ = 0.09); flowering and fruiting were greater in the population of the drained soil (Fig. 3). Flowering mainly occurred at the beginning of the year and fruiting occurred from March to September (Fig. 4). The two populations had greater leaf fall from the medium to the end of the dry season, but ripe leaves were still present (Fig. 4).
We also verified that the phenophases of Myrcia had no significant relation with any environmental variable in the wetland habitat (study objective 2, MMR test: F6.29, 21 = 0.41, p = 0.94, Wilk’s Λ = 0.08). However, the precipitation, maximum temperature and relative humidity influenced some phenophases of Myrcia in the drained habitat (study objective 2, MMR test: F5.03, 28 = 8.54, p = 0.01, Wilk’s Λ = 8.32e−07) (Table 1). We observed that the leaf budding and young leaf phenophases decreased as relative humidity increased (Online Appendix Fig. S1 a, b). The mature leaf phenophase increased with increasing relative humidity, and fruiting increased with an increase in precipitation (Online Appendix Fig. S1 c, d).
The phenophases of C. scabra in the wetland habitat also had a significant relation with some abiotic factors (study objective 2, MMR test: F5.03, 28 = 5.31, p = 0.03, Wilk’s Λ = 4.40e−06) (Table 1). Leaf budding increased and mature leaves decreased with an increase in the water table depth (Online Appendix Fig. S2 a, b). Fruiting was negatively influenced by an increase in the maximum temperature (Online Appendix Fig. S2 c).
We also recorded significant relations between the phenophases and abiotic factors in the C. scabra population in the drained habitat (study objective 2, MMR test: F5.03, 28 = 26.07, p < 0.001, Wilk’s Λ = 1.56e−08) (Table 1). Leaf budding and young leaves decreased with an increase in insolation (Online Appendix Fig. S3 a, b). Leaf fall decreased with an increase in temperature and relative humidity (Online Appendix Fig. S3 c, d). Flowering increased with temperature, and fruiting increased with relative humidity (Online Appendix Fig. S3 e, f).
Discussion
Unexpectedly, the congeneric species of Myrcia in the different habitats did not differ in their phenophases, but the C. scabra species did. This finding is interesting because in the case of differences between populations, we would expect that populations of the same species (Clethra) would have similar phenophases because closely related species are more ecologically similar (Burns and Strauss 2011).
The differences in the phenophases between C. scabra populations show that adaptation to the environment is an important issue. Moreover, this species may have phenotypic plasticity, meaning that it produces distinct phenotypes (as a function of its genotype) in different conditions (Padilla and Savedo 2013). Phenotypic plasticity is an important feature because it helps the organisms to adapt to new habitats and can contribute to genetic differentiation and speciation (Agrawal 2001). Specific studies, such as joint experiments in greenhouses (Sultan 2003), are necessary to prove that C. scabra really is phenotypically plastic.
At first, we thought that any differences in phenophases between habitats would be seen as smaller values for populations in the drained habitat because of water scarcity. However, flowering and fruiting phenophases of C. scabra were longer in the drained habitat. Studies have shown that roots in the upper layers of drier soils can penetrate deeper to take up water from deeper layers (Sharp and Davies 1985). Thus, C. scabra in the drained habitat might not be under hydric stress. In addition, C. scabra is known to inhabit different biomes (e.g., forests and grasslands) and can flourish even after fires (Freitas and Sazima 2006). This species seems to be quite resistant to various stresses (e.g., fires, dystrophic soils, high radiation), which allows it to inhabit several environments.
In ecological terms, the differences in the proportion of flowers and fruits between C. scabra populations may influence their population dynamics if such differences continue over time. For example, observations of pollinators revealed the probability that pollinators will alternate their visits between species that flower at the same time increases when flower colors of the two species are similar (Jager et al. 2011). Because there was a great proportion of flowers of the same species (of similar colors) in the drained soil, more pollinators may be attracted to those flowers and increase the plant reproductive success in the drained habitat. For the congeneric Myrcia species, pollinators would be attracted equally to both habitats because the mean number of flowers in the two populations were equal.
We predicted that the phenophases of the species in distinct habitats would be influenced by different factors but this only applied for C. scabra (one species of Myrcia was not influenced by any factor). Temperature was a relevant factor for both C. scabra populations. The effect of temperature on plant phenophases has been explored in many ecosystems (Caffarra and Donnelly 2011; Shen et al. 2011) and is gaining more attention because of increasing global warming (Ibáñez et al. 2010). In the present study, the temperature increase influenced some phenophases of C. scabra (leaf fall, flowering and fruiting) suggesting that future increases in global temperatures are likely to affect at least some phenophases of C. scabra.
We found that an increase in the water table depth increased the production of leaf budding but decreased mature leaves of C. scabra in the wetland habitat. This finding is opposite of a study on a wetland in Germany where changes in the water table did not affect the grassland phenology (Cornelius et al. 2014). An increase in insolation negatively affected the leaf budding and young leaves of C. scabra in the drained habitat, perhaps because insolation is directly related to temperature (Kaufmann et al. 2011) and the stress caused by high temperatures inhibits photosynthesis (Mathur et al. 2014). In addition, the soil in the drained habitat is drier, and water uptake should occur at greater depths.
The relative humidity was one of the main factors affecting the phenophases of Clethra and Myrcia in the drained habitat. A study on species in temperate regions showed that drier air significantly delays the start of the budburst in some arboreal species (Laube et al. 2014). Contrary to leaf budding and young leaves of tree species in temperate regions, leaf budding and young leaves of tropical trees may not benefit from high humidity because we verified that the increase in relative humidity was related to a decrease in leaf budding and young leaves of M. amazonica. Moreover, relative humidity has not been found to be an important trigger of changes in the reproductive phenophases of tropical species (Wright 1991).
With greater climate change, it is likely that known relationships or phenophases are also likely to change. Work in temperate regions has shown that climate change has affected the start of the tree growing season (Chmielewski and Rotzer 2001). Studies of this nature should inform planning strategies for the management and conservation of tropical forest species.
Conclusions
We verified that phenophases of congeneric Myrcia species in the wetland and drained habitats did not differ during the study but those of C. scabra in the two habitats did. C. scabra thus seems to have phenotypic plasticity, which should be further studied. Even in habitats near to each other, different factors affect the phenophases of the species: humidity, precipitation, insolation, and temperature were influential in the drained habitat, and temperature and water table depth were influential in the wetland. These findings help us better understand the role of habitat and abiotic factors on the phenophases of tropical tree species and are a starting point for designing conservation actions in a changing world.
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326. https://doi.org/10.1126/science.1060701
Alberto FJ, Derory J, Boury C, Frigerio JM, Zimmermann NE, Kremer A (2013) Imprints of natural selection along environmental gradients in phenology-related genes of Quercus petraea. Genetics 195:495–512. https://doi.org/10.1534/genetics.113.153783
Bartomeus I, Ascher JS, Wagner D, Bryan ND, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proc Natl Acad Sci 108:20645–20649. https://doi.org/10.1073/pnas.1115559108
Bencke CSC, Morellato LPC (2002) Comparative phenological study of nine tree species from three Atlantic forests, southeastern Brazil. Brazilian J Bot 25:237–248. https://doi.org/10.1590/S0100-84042002000200012
Bradley AV, Gerard FF, Barbier N, Weedon GP, Anderson LO, Huntingford C, Aragão LEOC, Zelazowski P, Arai E (2011) Relationships between phenology, radiation and precipitation in the Amazon region. Glob Chang Biol 17:2245–2260. https://doi.org/10.1111/j.1365-2486.2011.02405.x
Burns JH, Strauss SY (2011) More closely related species are more ecologically similar in an experimental test. Proc Natl Acad Sci 108:5302–5307. https://doi.org/10.1073/pnas.1013003108
Caffarra A, Donnelly A (2011) The ecological significance of phenology in four different tree species: effects of light and temperature on bud burst. Int J Biometeorol 55:711–721. https://doi.org/10.1007/s00484-010-0386-1
Cardoso FCG, Marques R, Botosso PC, Marques MCM (2012) Stem growth and phenology of two tropical trees in contrasting soil conditions. Plant Soil 354:269–281. https://doi.org/10.1007/s11104-011-1063-9
Chmielewski FM, Rotzer T (2001) Response of tree phenology to climate change across Europe. Agric For Meteorol 108:101–112. https://doi.org/10.1016/S0168-1923(01)00233-7
Climate-Data.org (2019) Climate: Ouro Preto. In: Climate-Data.Org. https://pt.climate-data.org/america-do-sul/brasil/minas-gerais/ouro-preto-765135/. Accessed 17 Jul 2019
Cornelius C, Heinichen J, Drösler M, Menzel A (2014) Impacts of temperature and water table manipulation on grassland phenology. Appl Veg Sci 17:625–635. https://doi.org/10.1111/avsc.12105
Cortés-Flores J, Hernández-Esquivel KB, González-Rodríguez A, Ibarra-Manríquez G (2017) Flowering phenology, growth forms, and pollination syndromes in tropical dry forest species: Influence of phylogeny and abiotic factors. Am J Bot 104:39–49. https://doi.org/10.3732/ajb.1600305
Davis JC (1986) Statistics and Data Analysis in Geology, 2nd edn. Wiley, Newyork
Davis CC, Willis CG, Primack RB, Miller-Rushing AJ (2010) The importance of phylogeny to the study of phenological response to global climate change. Philos Trans R Soc Lond B Biol Sci 365(1555):3202–3213. https://doi.org/10.1098/rstb.2010.0130
Davies TJ, Wolkovich EM, Kraft NJB, Salamin N, Allen JM, Ault TR, Betancourt JL, Bolmgren K, Cleland EE, Cook BI, Crimmins TM, Mazer SJ, McCabe GJ, Pau S, Regetz J, Schwartz MD, Travers SE (2013) Phylogenetic conservatism in plant phenology. J Ecol 101:1520–1530. https://doi.org/10.1111/1365-2745.12154
Ford C (2017) Getting started with Multivariate Multiple Regression. In: Univ. Virginia Libr. https://data.library.virginia.edu/getting-started-with-multivariate-multiple-regression/. Accessed 23 Mar 2020
Forrest J, Miller-Rushing AJ (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution. Philos Trans R Soc B Biol Sci 365:3101–3112. https://doi.org/10.1098/rstb.2010.0145
Freitas L, Sazima M (2006) Pollination biology in a tropical high-altitude grassland in Brazil: interactions at the community level. Ann Missouri Bot Gard 93:465–516. https://doi.org/10.3417/0026-6493(2007)93[465:PBIATH]2.0.CO;2
Fujaco MAG, Leite MGP, Messias MCTB (2010) Multitemporal analysis of land use changes in the Itacolomi State Park (MG) by geoprocessing techniques. Rem Rev Esc Minas 63:695–701. https://doi.org/10.1590/S0370-44672010000400016
Gordo O, Sanz JJ (2009) Long-term temporal changes of plant phenology in the Western Mediterranean. Glob Chang Biol 15:1930–1948. https://doi.org/10.1111/j.1365-2486.2009.01851.x
Gugger S, Kesselring H, Stöcklin J, Hamann E (2015) Lower plasticity exhibited by high- versus mid-elevation species in their phenological responses to manipulated temperature and drought. Ann Bot 116:953–962. https://doi.org/10.1093/aob/mcv155
Hammer Ø (2019) PAST 3.25—Reference Manual. In: Nat. Hist. Museum, Univ. Oslo. https://folk.uio.no/ohammer/past/past3manual.pdf. Accessed 3 Mar 2020
Hammer Ø, Harper DA, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Higuchi P, Oliveira-Filho AT, Silva AC, Machado ELM, santos RM, Pifano DS (2008) Tree community dynamics in a montane semi-deciduous tropical forest fragment in Lavras, Minas Gerais, on different soil classes. Rev Arvore 32:417–426. https://doi.org/10.1590/s0100-67622008000300004
Ibáñez I, Primack RB, Miller-Rushing AJ, Ellwood R, Higuchi H, Lee SD, Kobori H, Silander JA (2010) Forecasting phenology under global warming. Philos Trans R Soc B Biol Sci 365:3247–3260. https://doi.org/10.1098/rstb.2010.0120
IEF—Instituto Estadual de Florestas (2019) Parque Estadual do Itacolomi. In: Secretaria de Meio Ambiente de Minas Gerais. https://www.ief.mg.gov.br/component/content/193?task=view. Accessed 17 Jul 2019
Jager ML, Dreyer LL, Ellis AG (2011) Do pollinators influence the assembly of flower colours within plant communities? Oecologia 166:543–553. https://doi.org/10.1007/s00442-010-1879-7
Kaufmann RK, Kauppi H, Mann ML, Stock JH (2011) Reconciling anthropogenic climate change with observed temperature 1998–2008. Proc Natl Acad Sci 108:11790–11793. https://doi.org/10.1073/pnas.1102467108
Laube J, Sparks TH, Estrella N, Menzel A (2014) Does humidity trigger tree phenology? Proposal for an air humidity based framework for bud development in spring. New Phytol 202:350–355. https://doi.org/10.1111/nph.12680
Lorenzi H (1998) Brazilian trees I: Manual of identification and cultivation of tree plants in Brazil. Instituto Plantarum de Estudos da Flora, Nova Odessa
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol B Biol 137:116–126. https://doi.org/10.1016/J.JPHOTOBIOL.2014.01.010
Miller-Rushing AJ, Inouye DW, Primack RB (2008) How well do first flowering dates measure plant responses to climate change? The effects of population size and sampling frequency. J Ecol 96:1289–1296. https://doi.org/10.1111/j.1365-2745.2008.01436.x
Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858. https://doi.org/10.1038/35002501
Padilla DK, Savedo MM (2013) A systematic review of phenotypic plasticity in marine invertebrate and plant systems. Adv Mar Biol 65:67–94. https://doi.org/10.1016/B978-0-12-410498-3.00002-1
Pedreira G, de Sousa HC (2011) Tree community of a permanent flooded forest and its adjacent vegetation area in Ouro Preto, Minas Gerais state, Brasil. Ciência Florest 21:663–675. https://doi.org/10.5902/198050984511
Rodrigues RR (2004) A nomenclatural discussion of riparian forests. In: Rodrigues RR, de Leitão-Filho HF (eds) Matas ciliares: conservation and recovery, 3rd edn. Edusp/Fapesp, Sao Paulo, pp 91–99
Sharp RE, Davies WJ (1985) Root growth and water uptake by maize plants in drying soil. J Exp Bot 36:1441–1456. https://doi.org/10.1093/jxb/36.9.1441
Shen MG, Tang YH, Chen J, Zhu XL, Zheng YH (2011) Influences of temperature and precipitation before the growing season on spring phenology in grasslands of the central and eastern Qinghai-Tibetan Plateau. Agric For Meteorol 151:1711–1722. https://doi.org/10.1016/J.AGRFORMET.2011.07.003
Sobral M, Jarenkow JA, Brack P, Irgang B, Larocca J, Rodrigues R (2013) Arboreal and arborescent flora of Rio Grande do Sul, Brazil, 2nd edn. RiMa, San Carlos
Sultan SE (2003) Phenotypic plasticity in plants: a case study in ecological development. Evol Dev 5:25–33. https://doi.org/10.1046/j.1525-142X.2003.03005.x
USA National Phenology Network (2019) Phenophase. In: USA Natl. Phenol. Netw. https://usanpn.org/taxonomy/term/16. Accessed 21 Aug 2019
Walthall S, Lngram JA (1984) The investigation of aquifer parameters using multiple piezometers. Ground Water 22:25–30. https://doi.org/10.1111/j.1745-6584.1984.tb01472.x
Warton DI, Hui FKC (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology 92:3–10. https://doi.org/10.1890/10-0340.1
Weinfurt KP (1995) Multivariate analysis of variance. In: Grimm LG, Yarnold PR (eds) Reading and understanding multivariate statistics. American Psychological Association, Washington, pp 245–276
Wolkovich EM, Cook BI, Davies TJ (2014) Progress towards an interdisciplinary science of plant phenology: building predictions across space, time and species diversity. New Phytol 201:1156–1162. https://doi.org/10.1111/nph.12599
Wolkovich EM, Davies TJ, Schaefer H, Cleland E, Cook B, Travers S, Willis C, Davis C (2013) Temperature-dependent shifts in phenology contribute to the success of exotic species with climate change. Am J Bot 100:1407–1421. https://doi.org/10.3732/ajb.1200478
Wright SJ (1991) Seasonal drought and the phenology of understory shrubs in a tropical moist forest. Ecology 72:1643–1657. https://doi.org/10.2307/1940964
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the Research, Graduate and Innovation Department (PROPP) of the Federal University of Ouro Preto (UFOP).
The online version is available at http://www.springerlink.com.
Corresponding editor: Tao Xu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Londe, V., Pereira, J.A. & de Sousa, H.C. Phenological study of congeneric Myrcia species and Clethra scabra in wetland and drained habitats in a Montane Forest. J. For. Res. 32, 1419–1427 (2021). https://doi.org/10.1007/s11676-020-01216-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-020-01216-5