Abstract
The objective of this study was to compare eco-physiological and morphological parameters of a regionally endangered orchid species, Epipactis atrorubens (Hoffm. ex Bernh.) Bess., growing in two forest communities (on serpentine and granite outcrops) of the Middle Urals, Russia. Biodiversity, dominance, and phytocoenosis studies showed the colonization of a wide range of plant species on both sites. The physicochemical properties of the soil, chemical composition and morphological features of E. atrorubens, growing under technogenic conditions (asbestos deposits), on serpentine outcrops and in the natural environment of the granite massif were studied for the first time. The serpentine substrate differed from the granite one by its greater stoniness, circumneutral pH and lower contents of available nitrogen and phosphorus. Extremely high concentrations of magnesium were found in the serpentine soil, some 79 times higher than in the granite substrate. High concentrations of nickel (94 times), chromium (59 times), cobalt (17 times), and iron (4 times) were found in the serpentine substrate, higher than in the granite substrate. The differences between the sites for available metal contents and for root and shoot metal contents were significantly less. Concentrations of most of the metals in the roots were higher than in the shoots. Despite higher metal concentrations and lower nitrogen and phosphorus levels in serpentine soils, E. atrorubens had a larger population and greater viability compared to those growing on granite. Plants on serpentine outcrops were characterized by the formation of a larger number of fruits, greater root lengths and thicker leaf blades, compared to plants on granites. The well-developed orchid mycorrhizae contributed to the survival of this species under unfavorable serpentine conditions. Hence, serpentine outcrops formed due to the mining of asbestos could be a suitable substrate for the light-demanding E. atrorubens due to its capacity to adapt to dry, rocky, nutrient-depleted soils and limited competition from other plants.
Similar content being viewed by others
Introduction
The conservation of biodiversity is a current scientific and practical challenge. Anthropogenic activities lead to significant changes in vegetation cover and a reduction in the natural habitats of rare and endangered species, which results in a decrease in the number of individuals, and sometimes complete extinction (Jurkiewicz et al. 2001; Vakhrameeva et al. 2008; Swarts and Dixon 2009). To date, information has accumulated on the settlement of rare species, including some orchids, in man-made habitats (Jurkiewicz et al. 2001; Adamowski 2006; Rewicz et al. 2017). A comprehensive study of rare and endangered Orchidaceae genera and species, their biological and ecological features, the structure and dynamics of populations, and their association with other components of the community allows for the prediction of their development and improvement of protective measures (Tsiftsis et al. 2008; Vakhrameeva et al. 2008; Swarts and Dixon 2009).
An endangered orchid species of forest plant communities of the Middle Urals is E. atrorubens (Hoffm. ex Bernh.) Bess. (Mamaev et al. 2004; Red Book of Sverdlovsk Region 2008), which is also listed in the European Red List of Vascular Plants under the category of “Least Concern” (Bilz et al. 2011). This species is widely distributed in boreal, temperate and submeridional zones, mostly in the north to the subarctic, in the south to the Mediterranean, and in the east to Western Siberia and the Caucasus, excluding Eastern Siberia and the Far East (Buttler 1991; Delforge 2006). This species is found in deciduous, coniferous and mixed forests, along forest edges, and in shrub thickets with sunlight > 10% (Vakhrameeva et al. 2008). It prefers neutral or alkaline, dry or medium dry, well-aerated soils (Landolt 1977; Ellenberg et al. 1991). Epipactis atrorubens is a good colonizer on stony industrial deposits of talc, limestone, gypsum, zinc and mine tailings (Jurkiewicz et al. 2001; Adamowski 2006; Shefferson et al. 2008). However, low genetic variation can hinder the ability of this species to adapt to a new environment (Hens et al. 2017).
According to Delforge (2006) and Djordjević et al. (2016), E. atrorubens can also grow on serpentine areas. One such population was found recently in the Middle Urals on serpentine outcrops of asbestos deposits (Filimonova et al. 2019).
Serpentine soils are generally unfavorable for plant growth due to their poor physical and chemical properties (Kristy et al. 2005; Rajakaruna and Boyd 2014; Kumar et al. 2017). They have low nitrogen, phosphorus, potassium, and calcium contents, high levels of magnesium and iron, relatively high concentrations of nickel, chromium, cobalt, and pH from neutral to alkaline—which altogether, have detrimental and toxic effects on plants (Brady et al. 2005; Van der Ent et al. 2015; Kumar et al. 2017). Although numerous studies have been carried out on plants of serpentine areas (Kristy et al. 2005; Kazakou et al. 2010; Rajakaruna and Boyd 2014; Djordjević et al. 2016), adaptations of E. atrorubens, abundantly growing on serpentine outcrops, have been less studied. Therefore, a study of E. atrorubens colonizing serpentine deposits compared with the species from another forest community (granite outcrops), may make it possible to identify specific adaptations allowing this species to survive under stressful conditions. This will contribute to the development of measures for forest resource conservation.
This research aimed to: (1) analyze phytocoenotic conditions and physicochemical soil parameters of two forest plant communities of the Middle Urals (serpentine and granite outcrops); (2) compare the chemical composition (nitrogen, phosphorous and metal concentrations) of E. atrorubens colonizing these sites; and, (3) investigate the morphological features of this species and the degree of mycorrhizal association.
Materials and methods
Study site description
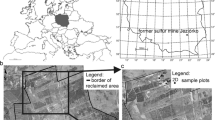
The research was carried out in the territory of the Middle Urals (a subzone of the southern taiga). Two naturally colonized populations of E. atrorubens were studied: P-1 on serpentine outcrops of the Shilovsky deposit (57°44′55″N, 60°12′38″E; altitude: 340 m); P-2 on the summit of Motaikha Mount (56°57′44″N, 60°19′46″E; altitude: 439 m), Fig. 1.
P-1 is located 132 km north of Ekaterinburg city, 2.5 km from Novoasbest village within the serpentine massif (Fig. 2a, b). The area is confined to lenticular deposits of talc-chlorite-carbonate rocks. Open cast mining operated from 1952 to 1992 for the extraction of fibrous asbestos. The average content of asbestos in the rocks was 4–5%. After the extraction of asbestos fibers, the waste materials were deposited along road sides, resulting in the formation of 2–3 tiered serpentine deposits. The height of the deposits is 30–35 m in an area of approximately 40 ha.
P-2 was found 50 km north-west of Ekaterinburg city in a forest plant community (Fig. 2c, d). The soils of this granite outcrop are primitive mountain brown forest soils. The area is experiencing a recreational load as it is situated next to a ski resort.
Biodiversity and dominance assessment
To determine the occurrence and the relative density of all species in each habitat, two transects were established, each with 25 quadrats (0.25 m2). The distance between each quadrat was 1.5 m and between transects 3 and 4 m. There were 50 quadrats in each habitat. Species diversity, projective coverage (the percent of ground area occupied by the species), and the number of individuals of each species were determined in each quadrat. In addition to grass species, tree/shrub shoots up to 40 cm were also recorded.
The assessment of alpha diversity was carried out using the index of species richness—the number of species calculated per unit area (0.25 m2).
Relative density (RD) is an estimate of the numerical strength of a species in relation to all other species, calculated as:
where Ni is the number of individuals of a species; Nall the number of individuals of all species.
Frequency (F) is the distribution or dispersion of an individual species, estimated as percentage of occurrence:
where Nqi is the number of quadrates in which the species occurred; Nqt the total number of quadrates.
Similarity in species composition between communities on serpentine and granite outcrops was calculated by Sorenson’s coefficient of community (Ks) (Smith and Smith 2012):
where C is number of common species in both habitats; S1 the number of species on the serpentine site; S2 the number of species on the granite site.
An analysis of community heterogeneity was carried out with Simpson’s Index for Dominance (D), calculated by the formula (Magurran 1988):
where ni is the number of individuals of each species; N the total number of individuals
To quantify species biodiversity, the Shannon–Wiener Diversity Index (H) was calculated (Mukhopadhyay et al. 2017):
where pi is the ratio between the number of individuals of each species to the total number of individuals (ni/N).
Pielou eveness index (E), an indicator of community equitability, was calculated based on the Shannon–Wiener index:
where H is Shannon–Wiener diversity index; S the total number of species; and ln the natural logarithm.
Sample collection and preparation
A 2000 m2 (100 × 20 m) area was selected on both sites with an area along the slope. Soil and plant samples were collected in August 2017 and 2018. Five 2 kg samples were collected from the 15-cm soil layer using a transect (Maiti 2013) and transferred for physicochemical analyses (granulometric, pH, available nitrogen, phosphorus, total and available metal concentrations). All samples were mixed thoroughly, air-dried for five days, oven-dried at 45 °C for 24 h and sieved. Due to legal restrictions, only four generative plant specimens (~ 40 to 50 cm length) were collected from each site. Each plant was excavated with soil to preserve the roots, placed in sterile bags, and delivered to the laboratory. The material was cut into shoot and root parts, washed by ultrasonication UM-4 (Unitra Unima, Poland), and finally with deionized water. After measuring the number and length of adventitious roots, 1–2-cm roots were used to study mycorrhiza parameters; the rest of the materials was used to determine nitrogen, phosphorus and metal concentrations. Before chemical analysis, soil and plant samples were dried for 4 h at 75 °C.
Physicochemical characteristics of serpentine and granite substrates
The first soil portion was used to determine percentage of different grain sizes and was carried out by standard sieve analysis (stones: > 10 mm; gravel large: 10–5 mm; gravel small: 5–3 mm; sand large: 3–1 mm; sand average: 1–0.25 mm; dust and clay: < 0.25 mm). The second portion was cleaned to separate stones, sieved through a < 2 mm mesh and the weight reduced to 1 kg by the coning-quartering method (Maiti 2013) and used to determine pH, nitrogen, phosphorus, and metal contents. The pH of soil–water suspension (1:2.5; w/v) was measured using Anion 4100 pH-meter (NPP Infraspak-Analit, Russia). The potentially available nitrogen content was determined according to the method of Cornfield (Prasad 1965). Available forms of phosphorus were extracted in a 0.2 N water solution of HCl and measured using UV–visible spectrophotometer Specord-40 (Analytik Jena AG, Germany) (Bekuzarova et al. 2016).
Total nitrogen and phosphorus contents
Total nitrogen and phosphorus contents in E. atrorubens shoots and roots were measured spectrophotometrically at 440 and 640 nm, respectively, after wet digestion of material with an acid mixture of HClO4 and H2SO4 (1:10; v/v). Total nitrogen was determined using Nessler’s reagent, whereas total phosphorus was determined by the standard ammonium molybdate in acid medium as described earlier (Borisova et al. 2014).
Metal analysis
Total metal concentrations in soil, shoots and roots were determined after wet digesting with concentrated analytical grade nitric acid using MARS 5 Digestion Microwave System (CEM Corporation, US). The available forms of metals were analyzed by heating the 5 g soil sample with 10 mL of 0.5 M nitric acid in a water bath and stirring for 3 h at 90 °C in compliance with standardized methodology (M-MVI-80 2008). Samples were then filtered into a 50 mL flask and the filter washed with 40 mL of 0.5 M nitric acid. All samples were prepared using double deionized Millipore water (Milli-Q system, Millipore, France). Magnesium, calcium, potassium, iron, zinc, copper, manganese, nickel, chromium, lead, and cobalt concentrations were determined using a flame atomic absorption spectrometer AA240FS (Varian Australia Pty Ltd, Australia). Standard reference materials (JSC Ural Chemical Reagents Plant, Russia) were used for the preparation and calibration of each analytical batch. Calibration coefficients were maintained at a high level of not less than 0.99. The bioconcentration factor (BCF) was calculated as the ratio of metal concentrations in shoots/roots to its available concentration in the soil. The translocation factor (TF) was calculated as the ratio of metal concentration in the shoots to its content in the roots.
Biometric assessment of E. atrorubens and mycorrhizal colonization
Fifteen flowering E. atrorubens plants growing on serpentine and granite outcrops were used to study shoot lengths, inflorescence, number of leaves, flowers and fruits, and total leaf area under in situ conditions.
Previously prepared roots of E. atrorubens were used for studying mycorrhizal association. Root tips up to 1.5 cm were cross sectioned to 20 μm with a freezing microtome MEP-01 (Technom, Russia). Root sections of 50 samples from each habitat were analyzed under a light microscope Meiji MT 4300L (Meiji Techno, Japan) at 100-x magnification. The presence of pelotons or intercellular hyphal coils in the root sections was determined and the proportion of sections with pelotons calculated. This parameter reflects the uniformity of distribution of the fungal symbiont in the plant root or the frequency of occurrence of the fungus.
Statistical analysis
Statistical analyses were carried out to determine mean values and standard errors (SE) of the data. Differences between physicochemical and morphological parameters of the two sites were determined with the non-parametric Mann–Whitney U-test. Different letters in tables and figures indicate significant differences (p < 0.05) between the two sites.
Results and discussion
Biodiversity, dominance, and phytocoenotic analysis
The serpentine site was up to 35–40% rocky with predominant trees species being Pinus sylvestris L., Betula pendula Roth, Populus tremula L., and Larix sibirica Ledeb., growing in small groups or solitary. Pinus sylvestris was 20–25 years-of-age and 6–8 m in height. A young Salix caprea L., S. cinerea L., Populus balsamifera L., P. suaveolens Fisch. community (1.5–2.0 height) was present at the edge of the site. Vegetation was sparse with 3–5% grasses. Plants such as Chamaecytisus ruthenicus (Fisch. ex Wołoszcz.) Klásková, Orthilia secunda (L.) House, Solidago virgaurea L. grew under the trees. Petrophytic species, plants that grow on rocky outcrops, such as Dendranthema zawadskii (Herbich) Tzvel., Dianthus versicolor Fisch. ex Link, Thymus talijevii Klok. Et Shost., Veronica spicata L., Rumex thyrsiflorus Fingerh., Euphrasia pectinate Ten. and ruderal species, ones first to colonize disturbed lands, such as Tussilago farfara L., Hordeum jubatum L., Melilotus albus Medik., were found in open areas.
On the second site, E. atrorubens was growing on the flat top of Motaikha Mount with outcrops of large blocks of granite, up to 15% of the surface. The top of the mountain was covered with P. sylvestris. Mature trees were partially cleared and in their place, a young (up to 15-years-old, 5–7 m high), mixed stand of P. sylvestris, B. pendula, L. sibirica had formed. The canopy density of the trees reached 60%. Plants such as Sorbus aucuparia L., Salix myrsinifolia Salisb., S. cinerea, Ch. ruthenicus formed the undergrowth. The total projective cover of grass and shrub layers at the second site varied from 10% to 80%. The predominant species were Calamagrostis arundinacea (L.) Roth, Vaccinium vitis-idaea L., Antennaria dioica (L.) Gaertn., Hieracium umbellatum L., Rubus saxatilis L., Polygonatum odoratum (Mill.) Druce. In addition, there was extensive moss-lichen cover.
A more detailed analysis of vegetation biodiversity was carried out on the species found on the site transects. A total of 22 species was identified on the serpentine outcrop and 34—on granite (Table S1). Only 12 species were common to both sites (Table 1). Sorenson’s coefficient of community (Ks) for the two E. atrorubens habitats was 0.4.
The distribution of species over the area was uniform for both sites. However, the E. atrorubens population P-1 (n = 305), was noticeably higher than P-2 (n = 36). The plant communities have equal Simpson’s indices for dominance (D = 0.3), which can be explained by the same number of dominant species with higher abundance. The low level of the dominance index underlines the importance of a small number of species in the structure of plant communities.
The greatest relative density and frequency on serpentine outcrops were noted for P. sylvestris, D. zawadskii, O. secunda and E. atrorubens, whereas on granites these were noted for A. dioica, V. vitis-idaea, C. arundinacea and P. sylvestris (Table 1, Table S1).
The quantitative assessment of biodiversity using the Shannon–Wiener Index (H) revealed a difference in plant communities on both sites. On serpentine outcrops, H = 2.5 was found compared to H = 3.0 for the granite site. The degrees of uniformity of E = 0.8 in the distribution of the participation of species were the same on both sites. The higher Shannon–Wiener indices of granite outcrops further indicates less favorable conditions for the growth of E. atrorubens.
This research has shown that plant communities are characterized by the dominance of a relatively few species. The P-1 population of E. atrorubens on the serpentine deposits has a higher size and density compared with the P-2. It is well known that orchids growing in secondary habitats are often abundant due to limited competition from other plants (Adamowski 2006). This research has shown that orchid population P-2 experienced phytocoenotic stress in which seed reproduction is difficult. This is confirmed by the increased Shannon–Wiener index indicating less favorable conditions for E. atrorubens on granite outcrops because of a competitive environment with other species.
Physicochemical characteristics of soils
Based on granulometric composition, serpentine substrates are mainly stony with 54–84% stones and gravel with diameters > 3.0 mm (Table 2). The rhizospheric soil zone of the granite massif was mainly sandy with sizes 0.25–3.0 mm, and the dust and clay fractions were 36%. The majority of the serpentine soil was dominated by skeletal part with sizes > 1.0 mm.
The pH of the serpentine soil was circumneutral (6.5–7.5) and the granite soil was weakly acidic. E. atrorubens prefers neutral or alkaline soils and avoids acidic soils. According to Vakhrameeva et al. (2008), the species mainly occurs in a pH range 7.5–9.0. However, populations of E. atrorubens in this study were growing both on a circumneutral (P-1) and on a weakly acidic substrate (P-2).
The serpentine soil had low available nitrogen and phosphorus levels, whereas in the granite soil, their concentrations were higher on average by 2.5 times (Table 2).
Therefore, the present study showed that a serpentine substrate differed from a granite one by greater stoniness and a circumneutral pH. Such conditions obviously better favored E. atrorubens growth on a serpentine habitat and contributed to an increase in P-1 population size.
Total nitrogen and phosphorus content in E. atrorubens
The levels of total nitrogen and phosphorus in E. atrorubens shoots were significantly higher (by 1.2 and 1.3 times, respectively) on granite substrates compared with plants from serpentine outcrops (Fig. 3). Based on nitrogen content in the roots, there were no significant differences between the populations, while phosphorus in plants on the serpentine substrate was 1.3 times lower than plants on granite one.
The conditions of mineral nutrition are of special importance for the development of the photosynthetic apparatus. Nitrogen is a part of all proteins, chlorophyll, nucleotides, amino acids, amides, and alkaloids; therefore, it is one of the principal elements of living organisms, whereas phosphorus is also important as it is a constituent of biomembranes and macromolecular structures and plays an important role in cell metabolism (Marschner 1995).
The lower content of nitrogen and phosphorus in E. atrorubens shoots and roots on serpentine outcrops may be explained by their lower concentration compared to levels on granite. However, some reports indicate the ability of this species to grow on infertile substrates (Landolt 1977; Ellenberg et al. 1991; Vakhrameeva et al. 2008). Under nutrient deficient conditions, mycorrhiza promote their mobilization and uptake by plant roots (Smith and Read 2008; Vakhrameeva et al. 2008). This is confirmed by higher phosphate contents in the roots (almost 2 times) compared with the shoots.
Metal content of serpentine and granite soils and E. atrorubens
A high concentration of total Mg (205 g kg−1) was found in the serpentine soil, some 79 times greater than in granite soil (Table 3). However, the difference in available Mg between the sites was less significant (23 times). The concentration of Mg in roots and shoots from the serpentine site was 8 times and 2 times higher than plants growing on the granite, respectively. Total and available Ca in the serpentine substrate was higher than in the granite (4 and 15 times, respectively). At the same time, levels in roots and shoots of E. atrorubens colonizing serpentine outcrops were slightly lower.
The difference between potassium concentrations among the two substrates was less noticeable and consecutively its content in the shoots was also the same (Table 3).
The bioconcentration factor (BCF) was in the order of K > Ca > Mg in both habitats. The BCF for potassium was similar in both sites (Table S2).
The ratio of total Mg:Ca in the serpentine substrate was about 15, whereas it was 1 in the granite substrate (Table 3). However, the ratio of their available forms on both the sites was close to one. Magnesium level in roots from the serpentine site was 2 times higher than calcium concentrations, while the reverse was found for shoots (Table 3). Average Ca concentration in roots and shoots from the granite habitat was 5 times higher than Mg.
Potassium and calcium levels in the shoots were higher than in roots (Table 3). This was confirmed by the translocation calculation, which was above one for the both habitats. At the same time, the plants differed significantly in magnesium translocation, which was two for the granite substrate and less than one for the serpentine substrate (Table S2).
High concentrations of total nickel, chromium, cobalt and iron were also found in the serpentine soil; the Ni content was 94 times, Cr—59 times, Co—17 times and Fe—4 times higher than in the granite soils. Nevertheless, available nickel in serpentine soils was 35 times and cobalt 8 times higher than in the granite soils, whereas iron and chromium level differences between sites averaged 20%. Differences between the sites on metal contents in roots and shoots were minor; average contents of nickel and chromium in plants on serpentine soil was about 4 times higher than plants on granite soil. Total and available lead, zinc and copper in granite soil was considerably higher than in the serpentine soil (Table 3).
The bioconcentration factors for shoots and roots of E. atrorubens colonizing a serpentine habitat were in the order of Zn > Cr > Cu > Pb > Ni (Fe) > Mn (Table S2). The BCF for the first four metals were greater than one, while the BCF for E. atrorubens on a granite soil were highest for Zn, Ni and Cr.
Levels of the metals in E. atrorubens roots were higher than in the shoots (Table 3). The metal translocation factor was below one (Table S2). The translocation factor for E. atrorubens colonizing the serpentine substrate was: Pb > Zn > Cu > Fe > Mn > Cr > Ni, while on the granite substrate it was Cr > Ni > Fe ≥ Mn > Pb > Zn > Cu.
Plants which are able to grow on serpentine soils show specific morphological and physiological adaptations, called “Syndrome” (Kazakou et al. 2008, 2010). Previously, it had also been reported that E. atrorubens grew luxuriantly on serpentine sites in Serbia without any detrimental effects (Djordjević et al. 2016).
This study shows that a possible reason for increased E. atrorubens tolerance to high metal concentrations in the serpentine soil is its ability to accumulate more metal in the root system and prevent its transfer to aboveground organs, suggesting its excluder strategy.
Biometric assessment of E. atrorubens and mycorrhizal association
Morphological analysis of two E. atrorubens populations showed no significant differences for shoot length and number of flowers per inflorescence between both sites (Table 4). This was found previously for leaf area (Filimonova et al. 2019). Leaves of plants growing on serpentine outcrops, as shown by this study, were significantly (about 2 times) thicker compared to plants growing on the granite substrate. This was due to an increase in the number of mesophyll cell layers and epidermal thickness in P-1 plants. The formation of a thicker leaf blade was the result of a higher light intensity and radiation in an open habitat. E. atrorubens is a light-demanding species (Vakhrameeva et al. 2008; Djordjević et al. 2016). An increase in leaf thickness may contribute to more efficient absorption and use of light energy in high insolation conditions. In addition, it could also compensate for the lack of photosynthetic pigments found previously (Filimonova et al. 2019).
The proportion of fruit formation was 74% on the serpentine outcrops, whereas it was significantly reduced (up to 11%) on the granite substrate (Table 4).
P-1 plants formed a better developed root system in comparison with plants on P-2. Average root numbers on the serpentine site was 59, with lengths up to 540 cm, whereas plants on granite outcrops averaged 24 roots with lengths of 180 cm.
Orchid mycorrhizae, represented by pelotons, were found in the root cells of E. atrorubens from both sites. In the early stages of development, E. atrorubens is an obligate mycosymbiotroph, whereas in the adult stage, the intensity of mycorrhizal association is mild to moderate and slightly depends on the fungi (Vakhrameeva et al. 2008; Tešitelová et al. 2012). E. atrorubens plants on granite outcrops had 82% pelotons (per unit root length), while it was only 50% on serpentine soil. The lesser numbers of root pelotons on serpentine soils may be associated with a lesser degree of plant community formation as well as with a high stony substrate which prevents the even distribution of the fungal mycelium. A smaller number of pelotons per unit root length of plants on serpentine substrate was compensated by longer root length.
Jurkiewicz et al. (2001) reported that mycorrhizae play an important role in the detoxification of metals in E. atrorubens roots and therefore, increase the resistance of the species to high metal concentrations in the environment. They have shown that heavy metals are stored in cell walls of fungi. This study shows an accumulation of most of the metals in the roots was significantly higher than in the shoots for both sites. It is possible that mycorrhizae make the E. atrorubens tolerant to high concentrations of heavy metals in serpentine soils.
On rich soils, E. atrorubens is strongly affected by more competitive species, which results in a decrease in population, seed productivity, and capacity to compete with other plant species (Vakhrameeva et al. 2008). In our studies, this was also evident by the less developed root system of individuals on granite substrate compared with plants on serpentine outcrops.
Conclusions
This research has revealed the main differences between the two forest plant communities. The degree of biodiversity, relative density and frequency of most of the species as well as the density of canopy and projective coverage of the grasses were higher on the granite site compared with the serpentine one. Therefore, the population of E. atrorubens growing on granite outcrops experiences phytocoenotic stress, in which seed reproduction is difficult. The population was noticeably lower. E. atrorubens colonizing the serpentine deposits were characterized by the formation of larger number of fruits, greater root lengths, and thicker leaf blades. They showed high viability despite lower nitrogen and phosphorus levels as well as higher metal concentrations compared to plants on the granite outcrop. The levels of most of the metals were considerably higher in the roots than in the shoots. The well-developed orchid mycorrhizae also obviously contributed to the survival of this species. Hence, serpentine outcrops formed due to the mining of asbestos could be a suitable substrate for the light-demanding E. atrorubens because of their ability to adapt to dry, rocky and nutrient-depleted soils and limited competition from other species. Future research will include identifying adaptive physiological mechanisms that contribute to the survival of orchids under extreme detrimental conditions of serpentine deposits.
References
Adamowski W (2006) Expansion of native orchids in anthropogenous habitats. Pol Bot Stud 22:35–44
Bekuzarova SA, Bome NA, Opalko AI, Weisfeld LI (2016) Temperate crop science and breeding ecological and genetic studies. Apple Academic Press, New York
Bilz M, Kell SP, Maxted N, Lansdown RV (2011) European red list of vascular plants. Publications Office of the European Union, Luxembourg
Borisova G, Chukina N, Maleva M, Prasad MNV (2014) Ceratophyllum demersum L. and Potamogeton alpinus Balb. from Iset’ river, Ural region, Russia differ in adaptive strategies to heavy metals exposure—a comparative study. Int J Phytoremediat 16:621–633
Brady KU, Kruckeberg AR, Bradshaw HD (2005) Evolutionary ecology of plant adaptation to soils. Annu Rev Ecol Evol Syst 36:243–266
Buttler PK (1991) Field guide to orchids of Britain and Europe. The Crowood Press, Swindon
Delforge P (2006) Orchids of Europe, North Africa and the Middle East. A & C Black, London
Djordjević V, Tsiftsis S, LakuŠić D, Stevanović V (2016) Niche analysis of orchids of serpentine and non-serpentine areas: implications for conservation. Plant Biosyst 150(4):710–719
Ellenberg H, Weber HE, Dull R, Wirth V, Werner W, Paulissen D (1991) Ecological indicator values of Central European plant species. Scr Geobot 18:1–248
Filimonova EI, Lukina NV, Glazyrina MA, Borisova GG, Maleva MG, Chukina NV (2019) Endangered orchid plant Epipactis atrorubens on serpentine and granite outcrops of Middle Urals, Russia: a comparative morphophysiological study. AIP Conf Proc 2063:040016. https://doi.org/10.1063/1.5087348
Hens H, Jäkäläniemi A, Tali K, Efimov P, Kravchenko AV, Kvist L (2017) Genetic structure of a regionally endangered orchid, the dark red helleborine (Epipactis atrorubens) at the edge of its distribution. Genetica 145(2):209–221
Jurkiewicz A, Turnau K, Mesjasz-Przybylowicz J, Przybylowicz W, Godzik B (2001) Heavy metal localisation in mycorrhizas of Epipactis atrorubens (Hoffm.) Besser (Orchidaceae) from zink mine tailings. Protoplasma 218:117–124
Kazakou E, Dimitrakopoulos PG, Baker AJM, Reeves RD, Troumbis AY (2008) Hypotheses, mechanisms and trade-offs of tolerance and adaptation to soils: from species to ecosystem level. Biol Rev 83:495–508
Kazakou E, Adamidis GC, Baker AJM, Reeves RD, Godino M, Dimitrakopoulos PG (2010) Species adaptation in soils in Lesbos Island (Greece): metal hyperaccumulation and tolerance. Plant Soil 332:369–385
Red Book of Sverdlovsk region: animals, plants, fungi (2008) Korytin NS (ed) Basko, Ekaterinburg (in Russian)
Kristy U, Kruckeberg A, Bradshaw H Jr (2005) Evolutionary ecology of plant adaptation to serpentine soils. Annu Rev Ecol Evol Syst 36:243–266
Kumar A, Maiti SK, Tripti Prasad MNV, Singh RS (2017) Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite-asbestos mine. J Soils Sed 17(5):1358–1368
Landolt E (1977) Okologische zeigerwerte zur schweizer flora. Veroff. Geobot. Inst. Rubel. H.64. Stiftung Riibel, Zurich
Magurran AE (1988) Ecological diversity and its measurement. Croom Helm, London
Maiti SK (2013) Ecorestoration of the coalmine degraded lands. Springer, New York
Mamaev SA, Knyazev MS, Kulikov PV, Filippov EG (2004) Orkhidnye Urala (Orchids of Ural), Ural. Otd. Ross. Akad. Nauk, Ekaterinburg (in Russian)
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
M-MVI-80 (2008) Methods for performing measurements of the mass fraction of elements in soil, ground and bottom sediment samples using atomic emission and atomic adsorption spectrometry. St. Petersburg (in Russian)
Mukhopadhyay S, Rana V, Kumar A, Maiti SK (2017) Biodiversity variability and metal accumulation strategies in plants spontaneously inhibiting fly ash lagoon. India. Environ Sci Pollut Res 24(29):22990–23005
Prasad R (1965) Determination of potentially available nitrogen in soils—a rapid procedure. Plant Soil 23(2):261–264
Rajakaruna N, Boyd RS (2014) Serpentine soils. In: Gibson D (ed) Oxford bibliographies in ecology. Oxford University Press, New York. https://doi.org/10.1093/obo/9780199830060-0055
Rewicz A, Bomanowska A, Shevera M, Kurowski J, Krason K, Zielinska K (2017) Cities and disturbed areas as man-made shelters for orchid communities. Not Bot Horti Agrobot Cluj Napoca 45(1):126–139
Shefferson R, Kull T, Tali K (2008) Mycorrhizal interactions of orchids colonizing Estonian mine tailings hills. Am J Bot 95(2):156–164
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Smith TM, Smith RL (2012) Elements of ecology, 9d edn. Pearson, Harlow
Swarts DN, Dixon WD (2009) Terrestrial orchid conservation in the age of extinction. Ann Bot 104:543–556
Tešitelová T, Tešitel J, Jersáková J, Říhová G, Selosse MS (2012) Simbiotic germination capability of four Epipactis species (Orchidaceae) is broader than expected from adult ecology. Am J Bot 99(6):1020–1032
Tsiftsis S, Tsiripidis I, Karagiannakidou V, Alifragis D (2008) Niche analysis and conservation of the orchids of east Macedonia (NE Greece). Acta Oecol 33:27–35
Vakhrameeva MG, Tatarenko IV, Varlygina TI, Torosyan GK, Zagulskii MN (2008) Orchids of Russia and adjacent countries (within the borders of the former USSR). A.R.G. Gantner, Ruggell
Van der Ent A, Rajakaruna R, Boyd RS, Echevarria G, Repin R, Williams D (2015) Global research on ultramafic (serpentine) ecosystems (8th international conference on serpentine ecology in Sabah, Malaysia): a summary and synthesis. Aust J Bot 63:1–16
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Project funding: The work was supported by the Ministry of Science and Higher Education of the Russian Federation in the framework of the State Task of UrFU No. 6.7696.2017.
The online version is available at http://www.springerlink.com
Corresponding editor: Tao Xu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Filimonova, E., Lukina, N., Glazyrina, M. et al. A comparative study of Epipactis atrorubens in two different forest communities of the Middle Urals, Russia. J. For. Res. 31, 2111–2120 (2020). https://doi.org/10.1007/s11676-019-01010-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-019-01010-y