Abstract
Summary
This study reported that the transitional zones in older adults were enlarged at the expense of the compact-appearing cortex with a greater porosity in all cortical sub-compartments. The magnitude of differences in areal and volumetric bone mineral density (aBMD, vBMD) between older and younger groups was similar.
Introduction
Aging is strongly associated with bone loss, but little is known about magnitudes of differences in bone microarchitectures, aBMD, and vBMD from peak bone mass (PBM) to senescence. We aimed to describe differences in aBMD, vBMD, and bone microarchitecture parameters at the distal radius between older and young adults.
Methods
We compared 201 participants, aged 62–89 years (female 47%) and 196 participants, aged 24–28 years (female 38%). Bone microarchitecture parameters at distal radius were measured using high-resolution peripheral computed tomography (HRpQCT). aBMD was measured using dual-energy X-ray absorptiometry (DXA). Unpaired t tests and chi-square tests were used to compare differences in means and proportions as appropriate.
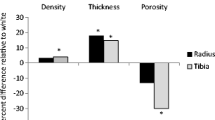
Results
Older adults had thinner compact-appearing cortices with larger (cross-sectional area: outer 30.96 mm2 vs. 28.38 mm2, inner 36.34 mm2 vs. 32.93 mm2) and thicker (outer 0.57 mm vs. 0.54 mm, inner 0.71 mm vs. 0.65 mm) transitional zones compared with young adults (all p < 0.05). Cortical porosity was modestly higher in older adults than in young adults (54% vs. 49%, p < 0.001). The magnitude of the difference in hip aBMD between older and young adults was slightly lower than of total radial vBMD (− 0.51 SD vs. − 0.78 SD).
Conclusion
Compared with young adults at the time of PBM, the transitional zones in older adults were enlarged at the expense of the compact-appearing cortex with a greater porosity in all cortical sub-compartments. The similar SD differences in aBMD and vBMD between older and younger groups suggest that the differences in bone area are not leading to major artefactual change in aBMD.


Similar content being viewed by others
References
Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA (1994) Symptomatic fracture incidence in elderly men and women-the Dubbo-osteoporosis-epidemiology-study (DOES). Osteoporos Int 4(5):277–282
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 353(9156):878–882
McGuigan FE, Murray L, Gallagher A, Davey-Smith G, Neville CE, Van’t Hof R et al (2002) Genetic and environmental determinants of peak bone mass in young men and women. J Bone Miner Res 17(7):1273–1279
Khosla S, Riggs BL (2005) Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin N Am 34(4):1015–1030
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 359(9321):1929–1936
Winzenberg T, Jones G (2011) Dual energy X-ray absorptiometry. Aust Fam Physician 40(1–2):43–44
Peacock DJ, Egger P, Taylor P, Cawley MI, Cooper C (1996) Lateral bone density measurements in osteoarthritis of the lumbar spine. Ann Rheum Dis 55(3):196–198
Hardcastle SA, Dieppe P, Gregson CL, Davey Smith G, Tobias JH (2015) Osteoarthritis and bone mineral density: are strong bones bad for joints? Bone Key Rep 4:624
Geusens P, Chapurlat R, Schett G, Ghasem-Zadeh A, Seeman E, De Jong J et al (2014) High-resolution in vivo imaging of bone and joints: a window to microarchitecture. Nat Rev Rheumatol 10(5):304–313
Biver E, Durosier-Izart C, Chevalley T, van Rietbergen B, Rizzoli R, Ferrari S (2018) Evaluation of radius microstructure and areal bone mineral density improves fracture prediction in postmenopausal women. J Bone Miner Res 33(2):328–337
Bjornerem A, Wang X, Bui M, Ghasem-Zadeh A, Hopper JL, Zebaze R et al (2018) Menopause-related appendicular bone loss is mainly cortical and results in increased cortical porosity. J Bone Miner Res 33(4):598–605
Bala Y, Bui QM, Wang XF, Iuliano S, Wang Q, Ghasem-Zadeh A, Rozental TD, Bouxsein ML, Zebaze RM, Seeman E (2015) Trabecular and cortical microstructure and fragility of the distal radius in women. J Bone Miner Res 30(4):621–629
Langsetmo L, Peters KW, Burghardt AJ, Ensrud KE, Fink HA, Cawthon PM, Cauley JA, Schousboe JT, Barrett-Connor E, Orwoll ES, Osteoporotic Fractures in Men (MrOS) Study Research Group (2018) Volumetric bone mineral density and failure load of distal limbs predict incident clinical fracture independent HR-pQCT BMD and failure load predicts incident clinical fracture of FRAX and clinical risk factors among older men. J Bone Miner Res 33(7):1302–1311
Yang Y, Pan F, Wu F, Squibb K, Thomson R, Winzenberg T, Jones G (2018) Familial resemblance in trabecular and cortical volumetric bone mineral density and bone microarchitecture as measured by HRpQCT. Bone. 110:76–83
Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ 3rd (2006) Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res 21(1):124–131
Dwyer T, Ponsonby AL, Newman NM, Gibbons LE (1991) Prospective cohort study of prone sleeping position and sudden infant death syndrome. Lancet. 337(8752):1244–1247
Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E (2013) A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 54(1):8–20
Zebaze R, Seeman E, Mbala A, Ghasemzadeh A, Mackie E, Bohte A. Method and system for image analysis of selected tissue structures. Google Patents; 2015
Nguyen T, Sambrook P, Kelly P, Jones G, Lord S, Freund J, Eisman J (1993) Prediction of osteoporotic fractures by postural instability and bone density. BMJ. 307(6912):1111–1115
Nguyen TV, Center JR, Eisman JA (2005) Femoral neck bone loss predicts fracture risk independent of baseline BMD. J Bone Miner Res 20(7):1195–1201
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM et al (2004) Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res 19(12):1945–1954
Henry YM, Fatayerji D, Eastell R (2004) Attainment of peak bone mass at the lumbar spine, femoral neck and radius in men and women: relative contributions of bone size and volumetric bone mineral density. Osteoporos Int 15(4):263–273
Rezaei A, Giambini H, Rossman T, Carlson KD, Yaszemski MJ, Lu L, Dragomir-Daescu D (2017) Are DXA/aBMD and QCT/FEA stiffness and strength estimates sensitive to sex and age? Ann Biomed Eng 45(12):2847–2856
Hillier TA, Cauley JA, Rizzo JH, Pedula KL, Ensrud KE, Bauer DC, Lui LY, Vesco KK, Black DM, Donaldson MG, Leblanc ES, Cummings SR (2011) WHO absolute fracture risk models (FRAX): do clinical risk factors improve fracture prediction in older women without osteoporosis? J Bone Miner Res 26(8):1774–1782
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349(4):327–334
Shanbhogue VV, Brixen K, Hansen S (2016) Age- and sex-related changes in bone microarchitecture and estimated strength: a three-year prospective study using HRpQCT. J Bone Miner Res 31(8):1541–1549
Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S (2008) A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J Bone Miner Res 23(2):205–214
Russo CR, Lauretani F, Seeman E, Bartali B, Bandinelli S, Di Iorio A et al (2006) Structural adaptations to bone loss in aging men and women. Bone. 38(1):112–118
Seeman E (2002) Pathogenesis of bone fragility in women and men. Lancet. 359(9320):1841–1850
Lauretani F, Bandinelli S, Griswold ME, Maggio M, Semba R, Guralnik JM, Ferrucci L (2008) Longitudinal changes in BMD and bone geometry in a population-based study. J Bone Miner Res 23(3):400–408
Kawalilak CE, Johnston JD, Cooper DM, Olszynski WP, Kontulainen SA (2016) Role of endocortical contouring methods on precision of HR-pQCT-derived cortical micro-architecture in postmenopausal women and young adults. Osteoporos Int 27(2):789–796
Bala Y, Zebaze R, Seeman E (2015) Role of cortical bone in bone fragility. Curr Opin Rheumatol 27(4):406–413
Kawalilak CE, Johnston JD, Olszynski WP, Kontulainen SA (2014) Characterizing microarchitectural changes at the distal radius and tibia in postmenopausal women using HR-pQCT. Osteoporos Int 25(8):2057–2066
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E (2010) Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 375(9727):1729–1736
Crisp A, Dixon T, Jones G, Cumming RG, Laslett LL, Bhatia K et al (2012) Declining incidence of osteoporotic hip fracture in Australia. Arch Osteoporos 7(1):179–185
Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, Kokolus SL, McMahon D, Lappe JM, Recker RR, Lang T, Guo XE (2010) Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25(10):2229–2238
Yang Y, Wu F, Antony B, Pan F, Winzenberg T, Jones G (2019) The association between first fractures sustained during childhood and adulthood and bone measures in young adulthood. J Pediatr 212:188–94.e2
Jones G, Cooley H (2002) Symptomatic fracture incidence in those under 50 years of age in southern Tasmania. J Paediatr Child Health 38(3):278–283
Levasseur R, Guaydier-Souquières G, Marcelli C, Sabatier J-P (2003) The absorptiometry T-score: influence of selection of the reference population and related considerations for everyday practice. Joint Bone Spine 70(4):290–293
Acknowledgments
We would like to thank the participants who made this study possible, and we gratefully acknowledge the role of TasOAC staff and volunteers in collecting the data.
Funding
This work was supported by the National Health and Medical Research Council (APP1045408).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Canchen Ma, Feng Pan, Yi Yang, Laura Laslett, Kathryn Squibb, Tania Winzenberg and Graeme Jones declare that they have no conflict of interest.
Roger Zebaze declares that he has no conflict of interest; he is a director of StraxCorp Pty Ltd.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ma, C., Pan, F., Yang, Y. et al. Distal radius bone microarchitecture: what are the differences between age 25 and old age?. Arch Osteoporos 15, 16 (2020). https://doi.org/10.1007/s11657-020-0696-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-020-0696-9