Abstract
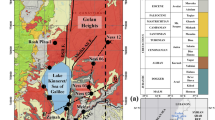
The origin of boron in boron-rich salt lakes in the Tibetan Plateau is highly controversial. In this study, we carried out a detailed study on boron geochemistry and isotope composition of lake sediments collected in Zigetang Co, central Tibet. Evaporites had high boron concentrations of 172.3–418.6 μg/g and δ11B values of −8.2‰ to −3.3‰, suggesting a non-marine origin for the saline lake. The boron isotopic fractionation factor, α, between evaporite and brackish water (αevaporite–brackish) decreased systematically with depth, from 0.9942 at the top of the drill core to 0.9893 at the bottom; the linear variation between αevaporite–brackish and depth reflects boron isotopic fractionation associated with progressive crystallization. The positive correlation between δ11B versus [B] and δ11B versus depth in the evaporite phase reflects pH and boron speciation in the solution control on the adsorption of boron, and B(OH)3 species incorporated preferentially into Mg(OH)2 precipitation at high pH.


Similar content being viewed by others
References
Chetelat B, Liu CQ, Gaillardet J, Wang QL, Zhao ZQ, Liang CS, Xiao YK (2009) Boron isotopes geochemistry of the Changjiang basin rivers. Geochim Cosmochim Acta 73:6084–6097
Hobbs MY, Reardon EJ (1999) Effect of pH on boron coprecipitation by calcite: further evidence for nonequilibrium partitioning of trace elements. Geochim Cosmochim Acta 63:1013–1021
Jin CF, Nther FG, Li SJ, Jia GD, Peng PA, Gleixner G (2016) Reduced early Holocene moisture availability inferred from D values of sedimentary n-alkanes in Zigetang Co, Central Tibetan Plateau. Holocene 26:556
Keren R, Gast RG, Baryosef B (1981) pH-Dependent boron adsorption by Na-Montmorillonite. Soil Sci Soc Am J 45:45–48
Palmer MR, Spivack AJ, Edmond JM (1987) Temperature and pH controls over isotopic fractionation during adsorption of boron on marine clay. Geochim Cosmochim Acta 51:2319–2323
Spivack AJ, Palmer MR, Edmond JM (1987) The sedimentary cycle of the boron isotopes. Geochim Cosmochim Acta 51:1939–1949
Umeda M, Iwata K, Obut OT, Sennikov NV, Izokh NG, Ermikov VD (1981) Boron adsorption by clay minerals using a phenomenological equation. Clays Clay Miner 29:198–204
Wei HZ, Jiang SY, Tan HB, Zhang WJ, Li BK, Yang TL (2014) Boron isotope geochemistry of salt sediments from the Dongtai salt lake in Qaidam Basin: boron budget and sources. Chem Geol 380:74–83
Xiao YK, Wang L (2006) An unusual isotopic fractionation of boron in synthetic calcium carbonate precipitated from seawater and saline water. Sci China Ser B Chem 49:454–465
Acknowledgements
This work was jointly supported by the National Basic Research Program (973 project) of China (2013CB956401), and the National Natural Science Foundation of China (Grant Nos. 41210004, 41661144042).
Author information
Authors and Affiliations
Corresponding authors
Additional information
11th International Symposium on Geochemistry of the Earth’s Surface.
Rights and permissions
About this article
Cite this article
Wang, X., Liu, C., Zhao, Z. et al. Boron isotope geochemistry of Zigetang Co saline lake sediments, Tibetan Plateau. Acta Geochim 36, 437–439 (2017). https://doi.org/10.1007/s11631-017-0185-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11631-017-0185-z