Abstract
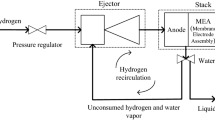
This work proposes a novel tubular structure of high-temperature proton exchange membrane fuel cell (PEMFC) integrated with a built-in packed-bed methanol steam reformer to provide hydrogen for power output. A two-dimensional axisymmetric non-isothermal model was developed in COMSOL Multiphysics 5.4 to simulate the performance of a tubular high temperature proton membrane fuel cell and a packed bed methanol reformer. The model considers the coupling multi-physical processes, including methanol reforming reaction, water gas shift reaction, methanol cracking reaction as well as the heat, mass and momentum transport processes. The sub-model of the tubular packed-bed methanol reformer is validated between 433 K and 493 K with the experimental data reported in the literature. The sub-model of the high temperature proton exchange fuel cell is validated between 393 K and 433 K with the published literature. Our results show that power output and temperature distribution of the integrated unit depend on methanol flow rates and working voltages. It was suggested that stable power generation performance of 0.14 W/cm2 and temperature drop in methanol steam reformer of ≤10 K could be achieved by controlling the methanol space-time ratio of ≥250 kg·s/mol with working voltage at 0.6 V, even in the absence of an external heat source.
Similar content being viewed by others
Abbreviations
- C :
-
Molar concentration/mol·m−3
- \(C_{\rm{S}}^{\rm{T}}\) :
-
Total surface concentrations of catalyst sites
- C p :
-
Specific heat capacity/J·kg−1·K−1
- CL:
-
Catalyst layer
- D :
-
Diffusion coefficient/m2·s−1
- d :
-
Diameter/m
- E a :
-
Activation energy/kJ
- F :
-
Faraday’s constant, 96 485 C·mol−1
- FC:
-
Fuel cell
- GDL:
-
Gas diffusion layer
- H :
-
Enthalpy/J·mol−1
- i :
-
Exchange current density/A·m−2
- j :
-
Transfer current density/A·m−3
- k :
-
Reaction kinetic constant/m2·s−1·mol−1
- k 0 :
-
Arrhenius pre-reaction factor/m2·s−1·mol−1
- lm:
-
Electrolyte volume fraction
- M :
-
Molecular weight/kg·mol−1
- Q :
-
Heat/J
- Q m :
-
Source term/kg·m−3·s−1
- R :
-
Universal gas constant, 8.314 J·mol−1·K−1
- r :
-
Reaction rate/mol·m−3·s−1
- S :
-
Source term, entropy/J·mol−1·K−1
- T :
-
Temperature/K
- U :
-
Electric potential/V
- u :
-
Superficial velocity/m·s−1
- V :
-
Voltage/V
- x :
-
Mole fraction
- α :
-
Transfer coefficient
- ε :
-
Porosity
- η :
-
Overpotential/V
- η conv :
-
Methanol conversion rate
- κ :
-
Permeability/m2
- λ :
-
Thermal conductivity/W·m−1·K−1
- µ :
-
Dynamic viscosity/kg·m−1·s−1
- ρ :
-
Density/kg·m−3
- σ :
-
Electrical conductivity/S·m−1
- φ :
-
Electric and ionic potential/V
- ω :
-
Mass fraction
- a:
-
Anode
- c:
-
Cathode
- cat:
-
Catalyst layer
- ele:
-
Electronic
- i,j :
-
Different species
- irr:
-
Irreversible
- m:
-
Membrane
- ohm:
-
Ohmic resistance
- ope:
-
Operational
- pro:
-
Protonic
- rev:
-
Reversible
- eff:
-
Effective value
- ref:
-
Reference state
References
Shen Y.T., Kwan T.H., Yao Q.H., et al., Performance numerical analysis of thermoelectric generator sizing for integration into a high temperature proton exchange membrane fuel cell. Applied Thermal Engineering, 2020, 178: 115486.
Herdem M.S., Sinaki M.Y., Farhad S., Hamdullahpur F., et al., An overview of the methanol reforming process: Comparison of fuels, catalysts, reformers, and systems. International Journal of Energy Research, 2019, 43(10): 5076–5105.
Chen C.Y., Lai W.H., Chen Y.K., et al., Characteristic studies of a PBI/H3PO4 high temperature membrane PEMFC under simulated reformate gases. International Journal of Hydrogen Energy, 2014, 39(25): 13757–13762.
Kim J., Kim M., Lee B.G., et al., Durability of high temperature polymer electrolyte membrane fuel cells in daily based start/stop operation mode using reformed gas. International Journal of Hydrogen Energy, 2015, 40(24): 7769–7776.
Authayanun S., Saebea D., Patcharavorachot Y., et al., Effect of different fuel options on performance of high-temperature PEMFC (proton exchange membrane fuel cell) systems. Energy, 2014, 68: 989–997.
Thomas S., Vang J.R., Araya S.S., et al., Experimental study to distinguish the effects of methanol slip and water vapour on a high temperature PEM fuel cell at different operating conditions. Applied Energy, 2017, 192: 422–436.
Schuller G., Vázquez F. V., Waiblinger W., et al., Heat and fuel coupled operation of a high temperature polymer electrolyte fuel cell with a heat exchanger methanol steam reformer. Journal of Power Sources, 2017, 347: 47–56.
Huang K.P., Lai W.H., Effects of anodic gas conditions on performance and resistance of a PBI/H3PO4 proton exchange membrane fuel cell with metallic bipolar plates. International Journal of Hydrogen Energy, 2017, 42(39): 24960–24967.
[9] Zhang C.Z., Zhou W.J., Ehteshami M.M., et al., Determination of the optimal operating temperature range for high temperature PEM fuel cell considering its performance, CO tolerance and degradation. Energy Conversion and Management, 2015, 105: 433–441.
Pan C., He R.H., Li Q.F., et al., Integration of high temperature PEM fuel cells with a methanol reformer. Journal of Power Sources, 2005, 145(2): 392–398.
Boaventura M., Alves I., Ribeirinha P., et al., The influence of impurities in high temperature polymer electrolyte membrane fuel cells performance. International Journal of Hydrogen Energy, 2016, 41(43): 19771–19780.
Ribeirinha P., Abdollahzadeh M., Sousa M.J., et al., Modelling of a high-temperature polymer electrolyte membrane fuel cell integrated with a methanol steam reformer cell. Applied Energy, 2017, 202: 6–19.
Ribeirinha P., Abdollahzadeh M., Pereira A., et al., High temperature PEM fuel cell integrated with a cellular membrane methanol steam reformer: Experimental and modelling. Applied Energy, 2018, 215: 659–669.
Israni S.H., Harold M.P., Methanol steam reforming in single-fiber packed bed Pd-Ag membrane reactor: Experiments and modeling. Journal of Membrane Science, 2011, 369(1): 375–387.
Sanz R., Calles J.A., Alique D., et al., Hydrogen production in a pore-plated Pd-membrane reactor: Experimental analysis and model validation for the water gas shift reaction. International Journal of Hydrogen Energy, 2015, 40(8): 3472–3484.
Hwang K., Oh D.K., Lee S.W., et al., Porous stainless steel support for hydrogen separation Pd membrane; fabrication by metal injection molding and simple surface modification. International Journal of Hydrogen Energy, 2017, 42(21): 14583–14592.
Pourmahmoud N., Sadeghifar H., Torkavannejad A., A novel, state-of-the-art tubular architecture for polymer electrolyte membrane fuel cells: Performance enhancement, size and cost reduction. International Journal of Heat and Mass Transfer, 2017, 108: 577–584.
Saghali Z., Mahmoudimehr J., Superiority of a novel conic tubular PEM fuel cell over the conventional cylindrical one. International Journal of Hydrogen Energy, 2017, 42(48): 28865–28882.
Sadiq Al-Baghdadi M.A.R., Three-dimensional computational fluid dynamics model of a tubular-shaped PEM fuel cell. Renewable Energy, 2008, 33(6): 1334–1345.
Bullecks B., Rengaswamy R., Bhattacharyya D., et al., Development of a cylindrical PEM fuel cell. International Journal of Hydrogen Energy, 2011, 36(1): 713–719.
Sierra J.M., Figueroa-Ramírez S.J., Díaz S.E., et al., Numerical evaluation of a PEM fuel cell with conventional flow fields adapted to tubular plates. International Journal of Hydrogen Energy, 2014, 39(29): 16694–16705.
Pourmahmoud N., Sadeghifar H., Torkavannejad A., A novel, state-of-the-art tubular architecture for polymer electrolyte membrane fuel cells: Performance enhancement, size and cost reduction. International Journal of Heat and Mass Transfer, 2017, 108: 577–584.
Saghali Z., Mahmoudimehr J., Superiority of a novel conic tubular PEM fuel cell over the conventional cylindrical one. International Journal of Hydrogen Energy, 2017, 42(48): 28865–28882.
Wang L., Tao Y.K., Zhang Z., et al., Molybdenum carbide coated 316L stainless steel for bipolar plates of proton exchange membrane fuel cells. International Journal of Hydrogen Energy, 2019, 44(10): 4940–4950.
Lee S.H., Kakati N., Maiti J., et al., Corrosion and electrical properties of CrN- and TiN-coated 316L stainless steel used as bipolar plates for polymer electrolyte membrane fuel cells. Thin Solid Films, 2013, 529: 374–379.
Bermúdez Agudelo M.C., Hampe M., Reiber T., et al., Investigation of porous metal-based 3D-printed anode GDLs for tubular high temperature proton exchange membrane fuel cells. Materials, 2020, 13(9): 2096.
Ribeirinha P., Abdollahzadeh M., Boaventura M., et al., H2 production with low carbon content via MSR in packed bed membrane reactors for high-temperature polymeric electrolyte membrane fuel cell. Applied Energy, 2017, 188: 409–419.
Nayebossadri S., Fletcher S., Speight J.D., et al., Hydrogen permeation through porous stainless steel for palladium-based composite porous membranes. Journal of Membrane Science, 2016, 515: 22–28.
Krastev V.K., Falcucci G., Jannelli E., et al., 3D CFD modeling and experimental characterization of HT PEM fuel cells at different anode gas compositions. International Journal of Hydrogen Energy, 2014, 39(36): 21663–21672.
Ahsan M.N., Paul C.P., Kukreja L.M., et al., Porous structures fabrication by continuous and pulsed laser metal deposition for biomedical applications; modelling and experimental investigation. Journal of Materials Processing Technology, 2011, 211(4): 602–609.
Jiao K., Alaefour I.E., Li X., Three-dimensional non-isothermal modeling of carbon monoxide poisoning in high temperature proton exchange membrane fuel cells with phosphoric acid doped polybenzimidazole membranes. Fuel, 2011, 90(2): 568–582.
Ju H., Investigation of the effects of the anisotropy of gas-diffusion layers on heat and water transport in polymer electrolyte fuel cells. Journal of Power Sources, 2009, 191(2): 259–268.
Ribeirinha P., Boaventura M., Lopes J.C.B., et al., Study of different designs of methanol steam reformers: Experiment and modeling. International Journal of Hydrogen Energy, 2014, 39(35): 19970–19981.
Peppley B.A., Amphlett J.C., Kearns L.M., et al., Methanol-steam reforming on Cu/ZnO/Al2O3 catalysts. Part 2. A comprehensive kinetic model. Applied Catalysis A: General, 1999, 179(1): 31–49.
Yin Y., Wang J.B., Yang X.L., et al., Modeling of high temperature proton exchange membrane fuel cells with novel sulfonated polybenzimidazole membranes. International Journal of Hydrogen Energy, 2014, 39(25): 13671–13680.
Huang H., Zhou Y.B., Deng H., et al., Modeling of high temperature proton exchange membrane fuel cell start-up processes. International Journal of Hydrogen Energy, 2016, 41(4): 3113–3127.
Sousa T., Mamlouk M., Scott K., An isothermal model of a laboratory intermediate temperature fuel cell using PBI doped phosphoric acid membranes. Chemical Engineering Science, 2010, 65(8): 2513–2530.
Shen Y., Kwan T.H., Yao Q.H., Performance numerical analysis of thermoelectric generator sizing for integration into a high temperature proton exchange membrane fuel cell. Applied Thermal Engineering, 2020, 178: 115486.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Shi, Y. & Cai, N. Modeling of Packed Bed Methanol Steam Reformer Integrated with Tubular High Temperature Proton Exchange Membrane Fuel Cell. J. Therm. Sci. 32, 81–92 (2023). https://doi.org/10.1007/s11630-022-1764-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11630-022-1764-9