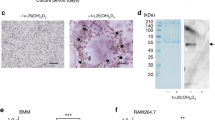
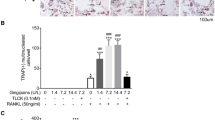
Abstract
Osteoblasts produce the receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin, the inducer and the suppressor of osteoclast differentiation and activation. We previously proposed that the degradation of osteoprotegerin by lysine-specific gingipain of Porphyromonas gingivalis and neutrophil elastase is one of the mechanisms of bone resorption associated with infection and inflammation. In the present study, we found that cathepsin K (CTSK) also degraded osteoprotegerin in an acidic milieu and the buffer with a pH of 7.4. The 37 k fragment of osteoprotegerin produced by the reaction with CTSK was further degraded into low molecular weight fragments, including a 13 k fragment, depending on the reaction time. The N-terminal amino acid sequence of the 37 k fragment matched that of the intact osteoprotegerin, indicating that CTSK preferentially hydrolyzes the death domain-like region of osteoprotegerin, not its RANKL-binding region. The 13 k fragment of osteoprotegerin was the C-terminal 13 k portion within the RANKL-binding region of the 37 k fragment. Finally, CTSK restored RANKL-dependent osteoclast differentiation that was suppressed by the addition of osteoprotegerin. Collectively, CTSK is a possible positive regulator of osteoclastogenesis.




Similar content being viewed by others
Data availability
The datasets of the current study are available from the corresponding author upon reasonable request.
References
Akiyama T, Miyamoto Y, Yoshimura K, Yamada A, Takami M, Suzawa T, Hoshino M, Imamura T, Akiyama C, Yasuhara R, Mishima K, Maruyama T, Kohda C, Tanaka K, Potempa J, Yasuda H, Baba K, Kamijo R (2014) Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-alpha and interleukin-1beta but suppresses that by interleukin-17A: importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J Biol Chem 289:15621–15630
Bonafe-Oliveira L, Faltin RM, Arana-Chavez VE (2003) Ultrastructural and histochemical examination of alveolar bone at the pressure areas of rat molars submitted to continuous orthodontic force. Eur J Oral Sci 111:410–416
Borel O, Gineyts E, Bertholon C, Garnero P (2012) Cathepsin K preferentially solubilizes matured bone matrix. Calcif Tissue Int 91:32–39
Brömme D, Okamoto K, Wang BB, Biroc S (1996) Human cathepsin O2, a matrix protein-degrading cysteine protease expressed in osteoclasts. Functional expression of human cathepsin O2 in Spodoptera frugiperda and characterization of the enzyme. J Biol Chem 271:2126–2132
Cawley KM, Bustamante-Gomez NC, Guha AG, MacLeod RS, Xiong J, Gubrij I, Liu Y, Mulkey R, Palmieri M, Thostenson JD, Goellner JJ, O’Brien CA (2020) Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep 32:108052
Cochran DL (2008) Inflammation and bone loss in periodontal disease. J Periodontol 79:1569–1576
Gowen M, Lazner F, Dodds R, Kapadia R, Field J, Tavaria M, Bertoncello I, Drake F, Zavarselk S, Tellis I, Hertzog P, Debouck C, Kola I (1999) Cathepsin K knockout mice develop osteopetrosis due to a deficit in matrix degradation but not demineralization. J Bone Miner Res 14:1654–1663
Heo SC, Kim YN, Choi Y, Joo JY, Hwang JJ, Bae MK, Kim HJ (2021) Elevated expression of cathepsin K in periodontal ligament fibroblast by inflammatory cytokines accelerates osteoclastogenesis via paracrine mechanism in periodontal disease. Int J Mol Sci 22:695
Ito H, Numabe Y, Sekino S, Murakashi E, Iguchi H, Hashimoto S, Sasaki D, Yaegashi T, Kunimatsu K, Takai H, Mezawa M, Ogata Y, Watanabe H, Hagiwara S, Izumi Y, Hiroshima Y, Kido J, Nagata T (2014) Evaluation of bleeding on probing and gingival crevicular fluid enzyme activity for detection of periodontally active sites during supportive periodontal therapy. Odontology 102:50–56
Kabasawa M, Ejiri S, Hanada K, Ozawa H (1996) Effect of age on physiologic and mechanically stressed rat alveolar bone: a cytologic and histochemical study. Int J Adult Orthodon Orthognath Surg 11:313–327
Kiviranta R, Morko J, Alatalo SL, NicAmjlaoibh R, Risteli J, Laitala-Leinonen T, Vuorio E (2005) Impaired bone resorption in cathepsin K-deficient mice is partially compensated for by enhanced osteoclastogenesis and increased expression of other proteases via an incrreased RANKL/OPG ratio. Bone 36:159–172
Kook SH, Jang YS, Lee JC (2011) Human periodontal ligament fibroblasts stimulate osteoclastogenesis in response to compression force through TNF-alpha-mediated activation of CD4+ T cells. J Cell Biochem 112:2891–2901
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129(469):e461–e432
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian YX, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Guo J, Delaney J, Boyle WJ (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Landzberg M, Doering H, Aboodi GM, Tenenbaum HC, Glogauer M (2015) Quantifying oral inflammatory load: oral neutrophil counts in periodontal health and disease. J Periodontal Res 50:330–336
Lv S, Liu H, Cui J, Hasegawa T, Hongo H, Feng W, Li J, Sun B, Kudo A, Amizuka N, Li M (2014) Histochemical examination of cathepsin K, MMP1 and MMP2 in compressed periodontal ligament during orthodontic tooth movement in periostin deficient mice. J Mol Histol 45:303–309
Novinec M, Kovačič L, Lenarčič B, Baici A (2010) Conformational flexibility and allosteric regulation of cathepsin K. Biochem J 429:379–389
Ochiai N, Nakachi Y, Yokoo T, Ichihara T, Eriksson T, Yonemoto Y, Kato T, Ogata H, Fujimoto N, Kobayashi Y, Udagawa N, Kaku S, Ueki T, Okazaki Y, Takahashi N, Suda T (2019) Murine osteoclasts secrete serine protease HtrA1 capable of degrading osteoprotegerin in the bone microenvironment. Commun Biol 2:86
Pilon JJ, Kuijpers-Jagtman AM, Maltha JC (1996) Magnitude of orthodontic forces and rate of bodily tooth movement. An experimental study. Am J Orthod Dentofacial Orthop 110:16–23
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19:1842–1844
Schneeweis LA, Willard D, Milla ME (2005) Functional dissection of osteoprotegerin and its interaction with receptor activator of NF-kappaB ligand. J Biol Chem 280:41155–41164
Scott DA, Krauss J (2012) Neutrophils in periodontal inflammation. Front Oral Biol 15:56–83
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Suda T, Jimi E, Nakamura I, Takahashi N (1997) Role of 1 alpha,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol 282:223–235
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Sugisaki R, Miyamoto Y, Yoshimura K, Sasa K, Kaneko K, Tanaka M, Itose M, Inoue S, Baba K, Shirota T, Chikazu D, Kamijo R (2020) Possible involvement of elastase in enhanced osteoclast differentiation by neutrophils through degradation of osteoprotegerin. Bone 132:115216
Tatara Y, Suto S, Itoh K (2017) Novel roles of glycosaminoglycans in the degradation of type I collagen by cathepsin K. Glycobiology 27:1089–1098
Tsukasaki M, Asano T, Muro R, Huynh NC, Komatsu N, Okamoto K, Nakano K, Okamura T, Nitta T, Takayanagi H (2020) OPG production matters where it happened. Cell Rep 32:108124
Udagawa N (2003) The mechanism of osteoclast differentiation from macrophages: possible roles of T lymphocytes in osteoclastogenesis. J Bone Miner Metab 21:337–343
White PC, Chicca IJ, Cooper PR, Milward MR, Chapple IL (2016) Neutrophil extracellular traps in periodontitis: a web of intrigue. J Dent Res 95:26–34
Whitty C, Wardale RJ, Henson FMD (2018) The regulation of sclerostin by cathepsin K in periodontal ligament cells. Biochem Biophys Res Commun 503:550–555
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K (1998a) Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 139:1329–1337
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998b) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Brömme D (2004) Cathepsin V, a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem 279:36761–36770
Yasuhara R, Miyamoto Y, Takami M, Imamura T, Potempa J, Yoshimura K, Kamijo R (2009) Lysine-specific gingipain promotes lipopolysaccharide- and active-vitamin D3-induced osteoclast differentiation by degrading osteoprotegerin. Biochem J 419:159–166
Funding
This work was supported by Grants-In-Aid for Scientific Research (KAKENHI) from the Japan Society for the Promotion of Science (21K09835, 22H03256).
Author information
Authors and Affiliations
Contributions
Kawai, Sugisaki, Yano, Sasa, and Minami performed experiments. Miyamoto designed the study. Maki and Kamijo analyzed the data. Kawai and Miyamoto prepared the original draft of the manuscript. Miyamoto, Maki, and Kamijo reviewed and edited the manuscript. All authors have read and agreed with the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawai, R., Sugisaki, R., Miyamoto, Y. et al. Cathepsin K degrades osteoprotegerin to promote osteoclastogenesis in vitro. In Vitro Cell.Dev.Biol.-Animal 59, 10–18 (2023). https://doi.org/10.1007/s11626-023-00747-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-023-00747-5