Abstract
Background
Antibiotics are often prescribed for hospitalized patients with chronic obstructive pulmonary disease (COPD) exacerbations. The use of procalcitonin (PCT) in the management of pneumonia has safely reduced antibiotic durations, but limited data on the impact of PCT guidance on the management of COPD exacerbations remain.
Objective
To determine the impact of PCT guidance on antibiotic utilization for hospitalized adults with exacerbations of COPD.
Design
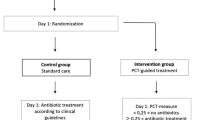
A retrospective, pre-/post-intervention cohort study was conducted to compare the management of patients admitted with COPD exacerbations before and after implementation of PCT guidance. The pre-intervention period was March 1, 2014, through October 31, 2014, and the post-intervention period was March 1, 2015, through October 31, 2015.
Participants
All patients with hospital admissions during the pre- and post-intervention period with COPD exacerbations were included. Patients with concomitant pneumonia were excluded.
Intervention
Availability of PCT laboratory values in tandem with a PCT guidance algorithm and education.
Main Measures
The primary outcome was duration of antibiotic therapy for COPD. Secondary objectives included duration of inpatient length of stay (LOS) and 30-day readmission rates.
Key Results
There were a total of 166 and 139 patients in the pre- and post-intervention cohorts, respectively. There were no differences in mean age (66.2 vs. 65.9; P = 0.82) or use of home oxygenation (34% vs. 39%; P = 0.42) in the pre- and post-intervention groups, respectively. PCT guidance was associated with a reduced number of antibiotic days (5.3 vs. 3.0; p = 0.01) and inpatient LOS (4.1 days vs. 2.9 days; P = 0.01). Respiratory-related 30-day readmission rates were unaffected (10.8% vs. 9.4%; P = 0.25).
Conclusions
Utilizing PCT guidance in the management of COPD exacerbations was associated with a decreased total duration of antibiotic therapy and hospital LOS without negatively impacting hospital readmissions.
Similar content being viewed by others
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a major cause of morbidity and mortality in the United States.1 There are approximately 12 million people in the US with COPD.2 Exacerbations of COPD cause over 800,000 hospitalizations in the US annually.2 COPD exacerbations are frequently caused by respiratory viruses, but can also be caused by bacterial infections and other non-infectious causes.3 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines state that antibiotics, when indicated, can shorten recovery time and hospitalization duration, while also reducing the risk of early relapse and treatment failure for patients with a COPD exacerbation.3 The guidelines recommend antibiotics should be given to patients with acute exacerbations who have three cardinal symptoms: increase in dyspnea, sputum volume, and sputum purulence; have two of the cardinal symptoms, if increased purulence of sputum is one of the two symptoms; or require mechanical ventilation.3
As a result of the guideline recommendations, antibiotics are often prescribed for hospitalized patients with COPD exacerbations.4 Interestingly, the European Society for Clinical Microbiology and Infectious Diseases describes non-pneumonic COPD exacerbations to be triggered primarily by viruses.5 Indeed, Lieberman and colleagues found patients with exacerbated COPD to be infected with viruses 46% of the time.6 Therefore, the common practice of prescribing antibiotics based on these subjective criteria is likely to lead to unnecessary overuse of antibiotics.4 As antibiotic resistance is becoming a serious threat to public health,7 antimicrobial stewardship programs (ASPs) have a significant opportunity to reduce antibiotic utilization in this commonly encountered disease state. One approach for ASPs is to utilize biomarkers able to distinguish bacterial causes from viral or non-infectious etiologies as the trigger for an episode of exacerbated COPD. Procalcitonin (PCT) is a biomarker specific to bacterial pathogens, and use of PCT-guided algorithms has demonstrated an ability to reduce antibiotic exposure in patients with pneumonia without negatively impacting clinical outcomes in randomized controlled studies.8,9 In response to bacterial-induced cytokines, PCT is released ubiquitously into the bloodstream.10 Conversely, production is attenuated by cytokines released in response to viral infections.11,12 Therefore, PCT helps distinguish between systemic bacterial infections and other inflammatory reactions or viral infections. There are few studies in the literature evaluating PCT in the management of COPD exacerbations.13,14 The goal of this study was to show the real-world impact of a PCT-guided algorithm on antibiotic utilization in adult patients hospitalized with COPD exacerbations.
PATIENTS AND METHODS
Study Setting and Population
The study took place at two teaching hospitals in Pittsburgh, Pennsylvania. Allegheny General Hospital (AGH) is a 631-bed quaternary care teaching facility with approximately 22,000 inpatient admissions yearly. The Western Pennsylvania Hospital (WPH) is a 317-bed community-based teaching hospital with nearly 6800 inpatient admissions annually. The quality assurance evaluation was granted exempt status from the Institutional Review Board.
Study Design
We conducted a retrospective, pre-/post-intervention study comparing the management of patients admitted with COPD exacerbations before and after implementation of a PCT guidance initiative. The pre-intervention period was March 1, 2014, through October 31, 2014, and the post-intervention period was March 1, 2015, through October 31, 2015.
Intervention
Our ASP worked with our microbiology laboratory to begin performing in-house PCT concentrations on March 1, 2015, in an effort to help distinguish bacterial etiologies from viral pathogens and non-infectious causes of COPD exacerbations and community acquired pneumonia. Our ASP created a clinical decision-making algorithm for suspected bacterial respiratory tract infections, including COPD exacerbations (Online Supplementary Fig. 1). The algorithm was approved by our ASP, Antimicrobial Subcommittee of the Pharmacy and Therapeutics Committee, and Pharmacy and Therapeutics Committee. For patients admitted with a COPD exacerbation, we recommended obtaining a PCT concentration within 24 h. For those with a concentration < 0.1 μg/l or 0.1–0.25 μg/l, initiation of antibiotic therapy was strongly discouraged and discouraged, respectively. If antibiotics were withheld, we recommended repeating PCT testing 6–24 h later. For patients with initial PCT concentrations 0.25–0.5 μg/l and > 0.5 μg/l, initiation of antibiotic therapy was recommended and strongly recommended, respectively. If antibiotic therapy was initiated, the recommended total duration was 5–7 days as indicated by the guidelines.3 The algorithm was implemented as a guide and antimicrobial prescribing was subject to the primary treatment team’s decision.
To inform the providers of our hospitals about the use of PCT, our ASP performed several educational steps. An electronic mail was sent from our Chair of Pathology, Director of Microbiology, and Medical Director of ASP to all house staff and medical staff notifying providers that PCT was available to order as a clinical tool while providing background information, clinical trial data, and the proposed role for PCT at AGH and WPH. We disseminated the clinical decision-making algorithm to all medical and house staff via electronic mail and incorporated it into our yearly Antimicrobial Guide, which is made available in print and on our network’s intranet. Laminated copies were posted at nursing units, physician work areas, the Emergency Department (ED), and the Internal Medicine residency department. A bi-folded 4” × 5” pocket card was created and distributed to the Internal Medicine residents. Lastly, we presented educational lectures to the Internal Medicine residency house staff, Internal Medicine medical staff, the Department of Hospitalist medicine, Department of Emergency medicine, Division of Pulmonary and Critical Care medicine, and the Division of Infectious Diseases.
Measurement of Serum PCT
PCT concentrations were measured using an automated heterogeneous sandwich immunoassay with fluorescence detection (VIDAS B.R.A.H.M.S. PCT assay; bioMérieux, Marcy L’Etoile, France). Total assay time was 20 min with a measuring range of 0.05 to 200 μg/l and a functional sensitivity of 0.09 μg/l.15
PCT was available in the Microbiology Laboratory 24 h per day and 7 days per week. Tests were run as needed. When ordered STAT from the AGH ED, results were available within 90 min. When ordered from other locations at AGH or WPH, results were made available during the same shift, usually within 6 h.
Data Collection
For the pre-intervention period, we identified all patients with a primary diagnosis of COPD exacerbation using the International Classification of Diseases, Ninth Revision (ICD-9), coding data. The search codes included acute bronchitis (466), chronic bronchitis (490–491), emphysema (492), and chronic airway obstruction (496) and ICD-10 codes: acute bronchitis (J20), unspecified chronic bronchitis (J42), emphysema (J34), and other chronic obstructive pulmonary disease (J44). These data were electronically extracted via our Quality Intelligence department.
For the post-intervention period, we included all patients who had a PCT concentration obtained within 24 h of admission with an admitting diagnosis of COPD. For patients with multiple hospitalizations, only the first admission was included. Demographic information, admission and discharge dates, and hospital length of stay (LOS) were extracted electronically via our Quality Intelligence department. Study investigators verified the admission diagnosis and obtained information regarding patient comorbidities, microbiologic data, radiographic studies, inpatient and outpatient antimicrobial therapy, and subsequent inpatient clinical encounters at AGH and WPH during the 30 days following hospital discharge via review of the electronic medical record.
Patients were excluded for age < 18 years, PCT concentration not obtained within 24 h of admission, transfer from an outside hospital, left against medical advice, death during index hospitalization, concomitant non-pulmonary bacterial infection that required antibiotic therapy, neutropenia, severe cell-mediated immunodeficiency, admission to the intensive care unit, receipt of mechanical ventilation, and identified to have pneumonia by imaging or if investigators were unable to determine the duration of antibiotics prescribed upon discharge.
Study Outcomes and Definitions
The primary outcome was to compare the duration of antibiotic therapy before and after the implementation of PCT guidance for the management of COPD exacerbations. Duration of therapy included in- and outpatient antibiotics prescribed to be administered.
Secondary outcomes included the duration of intravenous (IV) antibiotic treatment, duration of inpatient LOS, and all-cause hospital readmission as well as respiratory-related readmission within 30 days of discharge. In the post-intervention group, we compared duration of therapy for patients with low PCT concentrations (< 0.25 μg/l) versus patients with elevated PCT concentrations (≥ 0.25 μg/l). Another subset of the post-intervention group included only those with a low PCT concentration and compared inpatient length of stay and hospital readmission rates among patients who received 1 day or less of azithromycin to those who received > 1 day of azithromycin.
Severe immunodeficiency was defined as use of chronic immunosuppressive therapy at the time of admission (equivalent of >10 mg prednisone daily), human immunodeficiency virus with CD4 cell count < 350 cells/mm3, active malignancy with receipt of systemic chemotherapy within the 30 days prior to index admission, or receipt of prior solid organ transplant or hematopoietic stem cell transplantation.
Data Analysis
Differences between the pre- and post-intervention cohorts for continuous variables were assessed using the two-sample t-test. Differences in categorical data were assessed using chi-squared or Fisher’s exact test as appropriate. P < 0.05 was considered statistically significant. Stata statistical software, version 12, was used for data analysis.
RESULTS
There were a total of 166 and 139 patients in the pre- and post-intervention groups, respectively. Baseline demographics can be seen in Table 1.
The mean duration of antibiotic therapy decreased in the post-intervention group (5.3 vs. 3.0 days; P = 0.01) (Table 2). The number of patients who received 0–1 days of antibiotic therapy increased in the post-intervention group (14.5% vs. 43.8%; P = 0.01). Additionally, patients in the post-intervention group had a shorter duration of IV antibiotic therapy (2.5 vs. 1.9 days; P = 0.02) and a reduction of their hospital LOS (4.1 vs. 2.9 days; P = 0.01) (Table 2). There was no difference in hospital readmissions (14.5% vs. 16.6%; P = 0.25) or respiratory-related readmissions (10.8% vs. 9.4%; P = 0.18) between groups.
In the post-intervention cohort, no patients who were initially admitted to a general medical/surgical floor required transfer to an ICU later in the hospitalization. Additionally, no patients who were initially admitted to a general medical/surgical floor died during their hospitalization or in the 30 days following the index hospitalization. Also, in the post-intervention cohort, none of the patients who did not receive antibiotic therapy within the initial 48 h ended up receiving antibiotic therapy later in their hospital course.
In the post-intervention group, 11.5% (16/139) of patients had an elevated PCT (≥ 0.25 μg/l). Patients with an elevated PCT received longer durations of antibiotics compared to those with low PCT concentrations (5.3 vs. 2.7 days; P = 0.01). Other outcomes comparing this subset can be seen in Table 3. Among patients with a low PCT, 69 and 54 patients received 0–1 days of azithromycin and > 1 days of azithromycin therapy, respectively. In the group of 54 patients with a low PCT who received > 1 day of azithromycin, the mean duration of total antibiotic therapy was 4.7 days. They received a median of 5.0 days of antibiotic therapy with an interquartile range (IQR) of 3.0–6.0 days. There was no difference in readmission rates between those who received 0–1 days of azithromycin compared to those who received > 1 day of azithromycin therapy (17.4% vs. 18.5%; P = 0.87). There was a shorter hospital LOS in the patients who received 0–1 days of azithromycin (2.5 vs. 3.3 days; P = 0.03). Of patients who had an initial low PCT level in the post-intervention cohort, only 11 had a PCT level repeated within 24 h of the initial level. Of these 11 who had a repeat PCT, 10 had consecutive low levels, while only 1 who had an initial low level had a repeat level that was elevated within the next 24 h.
DISCUSSION
Our study showed that the implementation of PCT guidance for the management of COPD exacerbations was significantly associated with a reduction in the duration of antibiotic therapy. In this real-world experience, PCT utilization proved to be a useful tool to determine which patients should be prescribed antibiotics. Prior to PCT guidance, practitioners relied solely on the GOLD guidelines criteria, with one of the key components being sputum purulence, which may not be specific for identifying bacterial compared to viral etiologies.16 To quote the American College of Physicians and the Centers for Disease Control and Prevention, “Determining whether a patient has a viral or non-viral cause can be difficult. The presence of purulent sputum or a change in its color does not signify bacterial infection; purulence is due to the presence of inflammatory cells or sloughed mucosal epithelial cells.”16 Given that there was no difference in hospital readmissions between groups, we believe PCT guidance represents an objective criterion that can be safely utilized to reduce total antibiotic exposure for patients with this disease state.
Antibiotic use in exacerbated COPD is thought to decrease the hospital LOS.3 Interestingly, we found a decreased LOS in our post-intervention group of patients who received less antibiotic exposure by utilizing PCT guidance. This finding further solidifies the effectiveness of PCT guidance to safely decide which patients might benefit from antibiotic treatment.
Our study is unique in that it shows PCT guidance to be useful in a real-world practice when accompanied by a clinical decision-making algorithm. Other studies evaluating PCT guidance for patients with COPD exacerbations have primarily been in a controlled setting. One of those studies, the ProHOSP study, was a prospective randomized controlled trial that included patients with pneumonia and COPD exacerbations.14 Among the cohort with exacerbated COPD, there was a significant reduction in antibiotic exposure from 5.1 days to 2.5 days, without an increased incidence of combined adverse outcomes.14 A recent meta-analysis of 8 trials evaluating 1062 patients with acute exacerbated COPD found that PCT-based protocols decreased total antibiotic exposure by 3.83 days without affecting clinical outcomes such as rate of treatment failure, length of hospitalization, exacerbation recurrence rate, or mortality.17 All eight included studies were randomized or quasi-randomized controlled trials comparing PCT-based versus standard protocols to guide antibiotic use. Similar to these controlled trials, our real-world experience was associated with a reduction in antibiotic exposure for patients with this disease state without negatively affecting readmission rates. Additionally, in our study, only 11.5% of included patients in the post-intervention cohort had an elevated PCT level. This is consistent with prior studies that also found 20% or less of patients hospitalized with exacerbated COPD had an elevated PCT level.18,19 These consistent findings suggest that the majority of COPD exacerbations leading to hospitalization are due to non-bacterial etiologies.
The GOLD guidelines state that PCT guidance may not be a cost-effective test to utilize routinely for COPD exacerbations.3 However, a recent cost analysis of predicted outcomes in COPD exacerbation patients found PCT guidance to be cost effective.20 Indeed, the LOS and antibiotic reductions seen in our cohort would offset the costs associated with PCT concentrations.
An unanticipated finding of this study was the use of short course azithromycin despite a low PCT concentration in some patients. We hypothesize that this could be due to prescriber distrust of procalcitonin guidance or that azithromycin was used for its pleiotropic effects including anti-inflammatory properties. While maintenance dosing of azithromycin, 250 mg daily for 1 year, has shown a benefit in preventing hospital readmissions,21 there is no compelling evidence suggesting short-course azithromycin to have benefit. Our study showed no difference in readmissions and a shorter hospital LOS for those who were not prescribed azithromycin. However, the low number of patients in this subset analysis may not provide sufficient power to detect a difference. Further studies are needed to evaluate the utility of a short-course azithromycin treatment in patients who are hospitalized with exacerbated COPD and have a low PCT concentration.
Our current evaluation has several limitations. First, it was a retrospective analysis, and therefore we were limited to the data available in the electronic medical record. Additionally, patients in the pre-intervention cohort were identified by ICD-9 coding from hospital discharge data, and this may have led to an underestimation of the true number of hospitalized patients with COPD exacerbations. In the post-intervention group, it is possible that patients with severe COPD exacerbations did not have a PCT concentration obtained as the treating physician would have utilized antibiotics regardless of PCT result and therefore would have not been identified for our analysis. Furthermore, a paucity of patients had a PCT level repeated in our post-intervention cohort. Additionally, readmission data were limited to AGH and WPH. Therefore, visits to other inpatient facilities, urgent care centers, and physicians’ outpatient offices may have been missed. Lastly, our current analysis was only designed to evaluate clinically stable patients admitted with acute exacerbated COPD. Our institutional algorithm was not designed or intended to withhold antibiotics from patients with hemodynamic or respiratory instability. As our study aim was to evaluate the impact of this institutional PCT guidance algorithm, we only were able to evaluate the impact of this algorithm on hospitalized stable patients. Thus, we excluded patients admitted to an intensive care unit or who were placed upon mechanical ventilation upon their admission. Therefore, our findings cannot be extrapolated to unstable patients with more severe COPD exacerbations.
CONCLUSIONS
Patients with COPD exacerbations requiring hospitalization have a high readmission rate, and additional antibiotic exposures are likely.2,4 The repeated exposure of antibiotics in this patient population can lead to antimicrobial resistance, which is a serious threat to public health.7 Our study demonstrates that the implementation of PCT guidance as part of a clinical decision-making algorithm is associated with reduced antibiotic exposures without negatively impacting hospital readmissions in the management of adult patients admitted with COPD exacerbations.
References
Mannino DM, Kiriz VA. Changing the burden of COPD mortality. Int J Chron Obstruct Pulmon Dis. 2006:1(3):219-233.
Wier LM, Elixhauser A, Pfuntner A, Au DH. Overview of hospitalizations among patients with COPD, 2008: Statistical brief #106. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville: Agency for Healthcare Research and Quality (US); 2006.
Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017: 195(5):557-582.
Lopez-Campos JL, Hartl S, Pozo-Rodriguez F, Roberts CM. Antibiotic Prescription for COPD Exacerbations Admitted to Hospital: European COPD Audit. PLoS One. 2015:10(4):e0124374.
Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect. 2011:17 Suppl 6:E1-59.
Lieberman D, Lieberman D, Gelfer Y, et al. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest. 2002:122(4):1264-1270.
Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009:48(1):1-12.
Gogos CA, Drosou E, Bassaris HP, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000:181(1):176-180.
Haeuptle J, Zaborsky R, Fiumefreddo R, et al. Prognostic value of procalcitonin in Legionella pneumonia. Eur J Clin Microbiol Infect Dis. 2009:28(1):55-60.
Muller B, White JC, Nylen ES, Snider RH, Becker KL, Habener JF. Ubiquitous expression of the calcitonin-i gene in multiple tissues in response to sepsis. J Clin Endocrinol Metab. 2001:86(1):396-404.
Christ-Crain M, Muller B. Biomarkers in respiratory tract infections: diagnostic guides to antibiotic prescription, prognostic markers and mediators. Eur Respir J. 2007:30(3):556-573.
Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology. 2005:146(6):2699-2708.
Schuetz P, Muller B, Christ-Crain M, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012(9):Cd007498.
Schuetz P, Christ-Crain M, Thomann R, et al. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009:302(10):1059-1066.
VIDAS B.R.A.H.M.S. Procalcitonin technical details. bioMérieux website. http://www.biomerieux-diagnostics.com/vidas-brahms-pct. Accessed Dec 14 2017.
Harris AM, Hicks LA, Qaseem A. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016:164(6):425-434.
Mathiodakis AG, Chatzimavridou-Grigoriadou V, Corlateanu A, et al. Procalcitonin to guide antibiotic administration in COPD exacerbations: a meta-analysis. Eur Respir Rev. 2017:26:160073.
Verduri A, Luppi F, D’Amico R, et al. Antibiotic treatment of severe exacerbations of chronic obstructive pulmonary disease with procalcitonin: a randomized noninferiority trial. PLoS One. 2015;10:e0118241.
Stolz D, Christ-Crain M, Bingisser R, et al. Antibiotic treatment of exacerbations of COPD: a randomized, controlled trial comparing procalcitonin-guidance with standard therapy. Chest. 2007;131:9-19.
Van der Maas ME, Mantjes G, Steuten LM. Procalcitonin biomarker algorithm reduces antibiotic prescriptions, duration of therapy, and costs in chronic obstructive pulmonary disease: a Comparison in The Netherlands, Germany, and the United Kingdom. OMICS. 2017:21(4):232-243.
Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011:365(8):689-698.
Funding
This research received no specific grant from any funding agency in the public commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The authors thank Karen Kasarda for assistance with implementation of procalcitonin guidance in the microbiology laboratory at Allegheny General Hospital.
Corresponding author
Ethics declarations
Prior Presentations
This work was presented at ID Week, October 7, 2017.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Electronic supplementary material
Fig S1
(GIF 98 kb)
Rights and permissions
About this article
Cite this article
Bremmer, D.N., DiSilvio, B.E., Hammer, C. et al. Impact of Procalcitonin Guidance on Management of Adults Hospitalized with Chronic Obstructive Pulmonary Disease Exacerbations. J GEN INTERN MED 33, 692–697 (2018). https://doi.org/10.1007/s11606-018-4312-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-018-4312-2