Abstract
Background
Conflicting data exist on predictors of nodal metastases and their impact on survival in patients with pancreatic neuroendocrine tumors (PNETs). We aim to identify factors associated with lymph node involvement and evaluate the effect of nodal metastases on survival.
Methods
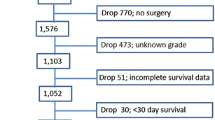
All patients undergoing surgery for PNETs in the Surveillance, Epidemiology, and End Results (SEER) tumor registry from 1988 to 2010 were included. Predictors of lymph node involvement and disease-specific survival (DSS) were evaluated using logistic regression and Cox regression, respectively.
Results
Patients (1,915) underwent surgery for a PNET (62 % nonfunctional). Nodal positivity was associated with increasing tumor size (p < 0.001) and grade (p < 0.001). Unadjusted DSS at 5 years was 81 % for N0, 74 % for Nx, and 69 % for N1, respectively, (p < 0.001). After adjustment for tumor size and grade, DSS was significantly decreased in N1 patients (HR 1.57; 95 % CI 1.23–1.95). For patients who had at least one node examined and had low-grade PNETs <1 cm, no nodal metastases were found.
Conclusions
High tumor grade and increasing size predict nodal metastases in patients with PNETs. N1 status is independently associated with decreased DSS. Low-grade tumors <1 cm may be observed or enucleated.


Similar content being viewed by others
References
Yao, J.C., M. Hassan, A. Phan, C. Dagohoy, C. Leary, J.E. Mares, E.K. Abdalla, J.B. Fleming, J.N. Vauthey, A. Rashid and D.B. Evans, One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol, 2008. 26(18): p. 3063–72.
de Herder, W.W., Biochemistry of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab, 2007. 21(1): p. 33–41.
Modlin, I.M., K. Oberg, D.C. Chung, R.T. Jensen, W.W. de Herder, R.V. Thakker, M. Caplin, G. Delle Fave, G.A. Kaltsas, E.P. Krenning, S.F. Moss, O. Nilsson, G. Rindi, R. Salazar, P. Ruszniewski and A. Sundin, Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol, 2008. 9(1): p. 61–72.
Kloppel, G., A. Perren and P.U. Heitz, The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci, 2004. 1014: p. 13–27.
Bosman, F.T., World Health Organization and International Agency for Research on Cancer, WHO classification of tumours of the digestive system. 4th ed. World Health Organization classification of tumours. 2010, Lyon: International Agency for Research on Cancer. 417 p.
Rindi, G., G. Kloppel, H. Alhman, M. Caplin, A. Couvelard, W.W. de Herder, B. Erikssson, A. Falchetti, M. Falconi, P. Komminoth, M. Korner, J.M. Lopes, A.M. McNicol, O. Nilsson, A. Perren, A. Scarpa, J.Y. Scoazec and B. Wiedenmann, TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch, 2006. 449(4): p. 395–401.
Boyar Cetinkaya, R., M. Vatn, L. Aabakken, D.S. Bergestuen and E. Thiis-Evensen, Survival and prognostic factors in well-differentiated pancreatic neuroendocrine tumors. Scand J Gastroenterol, 2014. 49(6): p. 734–41.
Cherenfant, J., S.J. Stocker, M.K. Gage, H. Du, T.A. Thurow, M. Odeleye, S.W. Schimpke, K.L. Kaul, C.R. Hall, I. Lamzabi, P. Gattuso, D.J. Winchester, R.W. Marsh, K.K. Roggin, D.J. Bentrem, M.S. Baker, R.A. Prinz and M.S. Talamonti, Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery, 2013. 154(4): p. 785–91; discussion 791–3.
Bilimoria, K.Y., D.J. Bentrem, R.P. Merkow, J.S. Tomlinson, A.K. Stewart, C.Y. Ko and M.S. Talamonti, Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg, 2007. 205(4): p. 558–63.
Ito, H., M. Abramson, K. Ito, E. Swanson, N. Cho, D.T. Ruan, R.S. Swanson and E.E. Whang, Surgery and staging of pancreatic neuroendocrine tumors: a 14-year experience. J Gastrointest Surg, 2010. 14(5): p. 891–8.
Han, X., X. Xu, D. Jin, D. Wang, Y. Ji and W. Lou, Clinicopathological characteristics and prognosis-related factors of resectable pancreatic neuroendocrine tumors: a retrospective study of 104 cases in a single Chinese center. Pancreas, 2014. 43(4): p. 526–31.
Partelli, S., S. Gaujoux, L. Boninsegna, R. Cherif, S. Crippa, A. Couvelard, A. Scarpa, P. Ruszniewski, A. Sauvanet and M. Falconi, Pattern and clinical predictors of lymph node involvement in nonfunctioning pancreatic neuroendocrine tumors (NF-PanNETs). JAMA Surg, 2013. 148(10): p. 932–9.
Sarmiento, J.M., M.B. Farnell, F.G. Que and D.M. Nagorney, Pancreaticoduodenectomy for islet cell tumors of the head of the pancreas: long-term survival analysis. World J Surg, 2002. 26(10): p. 1267–71.
Scarpa, A., W. Mantovani, P. Capelli, S. Beghelli, L. Boninsegna, R. Bettini, F. Panzuto, P. Pederzoli, G. delle Fave and M. Falconi, Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol, 2010. 23(6): p. 824–33.
Tomassetti, P., D. Campana, L. Piscitelli, R. Casadei, D. Santini, F. Nori, A.M. Morselli-Labate, R. Pezzilli and R. Corinaldesi, Endocrine pancreatic tumors: factors correlated with survival. Ann Oncol, 2005. 16(11): p. 1806–10.
Toste, P.A., B.E. Kadera, S.F. Tatishchev, D.W. Dawson, B.M. Clerkin, R. Muthusamy, R. Watson, J.S. Tomlinson, O.J. Hines, H.A. Reber and T.R. Donahue, Nonfunctional pancreatic neuroendocrine tumors <2 cm on preoperative imaging are associated with a low incidence of nodal metastasis and an excellent overall survival. J Gastrointest Surg, 2013. 17(12): p. 2105–13.
Bettini, R., L. Boninsegna, W. Mantovani, P. Capelli, C. Bassi, P. Pederzoli, G.F. Delle Fave, F. Panzuto, A. Scarpa and M. Falconi, Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol, 2008. 19(5): p. 903–8.
Haynes, A.B., V. Deshpande, T. Ingkakul, P.A. Vagefi, J. Szymonifka, S.P. Thayer, C.R. Ferrone, J.A. Wargo, A.L. Warshaw and C. Fernandez-del Castillo, Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg, 2011. 146(5): p. 534–8.
Rindi, G., M. Falconi, C. Klersy, L. Albarello, L. Boninsegna, M.W. Buchler, C. Capella, M. Caplin, A. Couvelard, C. Doglioni, G. Delle Fave, L. Fischer, G. Fusai, W.W. de Herder, H. Jann, P. Komminoth, R.R. de Krijger, S. La Rosa, T.V. Luong, U. Pape, A. Perren, P. Ruszniewski, A. Scarpa, A. Schmitt, E. Solcia and B. Wiedenmann, TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. J Natl Cancer Inst, 2012. 104(10): p. 764–77.
Kaplan, E.L. and P. Meier, Nonparametric Estimation from Incomplete Observations Journal of the American Statistical Association, 1958. 53(282): p. 457–481.
Hashim, Y.M., K.M. Trinkaus, D.C. Linehan, S.S. Strasberg, R.C. Fields, D. Cao and W.G. Hawkins, Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg, 2014. 259(2): p. 197–203.
Hasegawa, T., K. Yamao, S. Hijioka, V. Bhatia, N. Mizuno, K. Hara, H. Imaoka, Y. Niwa, M. Tajika, S. Kondo, T. Tanaka, Y. Shimizu, T. Kinoshita, T. Kohsaki, I. Nishimori, S. Iwasaki, T. Saibara, W. Hosoda and Y. Yatabe, Evaluation of Ki-67 index in EUS-FNA specimens for the assessment of malignancy risk in pancreatic neuroendocrine tumors. Endoscopy, 2014. 46(1): p. 32–8.
Weynand, B., I. Borbath, V. Bernard, C. Sempoux, J.F. Gigot, C. Hubert, V. Lannoy, P.H. Deprez and A. Jouret-Mourin, Pancreatic neuroendocrine tumour grading on endoscopic ultrasound-guided fine needle aspiration: high reproducibility and inter-observer agreement of the Ki-67 labelling index. Cytopathology, 2013. doi: 10.1111/cyt.12111
Katanuma, A., H. Maguchi, K. Yane, S. Hashigo, T. Kin, M. Kaneko, S. Kato, R. Kato, R. Harada, M. Osanai, K. Takahashi and M. Nojima, Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci, 2013. 58(7): p. 2093–9.
Kishi, Y., K. Shimada, S. Nara, M. Esaki, N. Hiraoka and T. Kosuge, Basing Treatment Strategy for Non-functional Pancreatic Neuroendocrine Tumors on Tumor Size. Ann Surg Oncol, 2014. p 28882–8
Manasanch, E.E., J.K. Smith, A. Bodnari, J. McKinney, C. Gray, T.P. McDade and J.F. Tseng, Tumor registry versus physician medical record review: a direct comparison of patients with pancreatic neuroendocrine tumors. J Oncol Pract, 2011. 7(2): p. 111–6.
Grant Support
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Marshall Baker: Pancreatic neuroendocrine tumors are rare entities with substantial biologic variability. Studies employing national data sets represent the best methods currently available to develop and evaluate clinical approaches to these rare and unpredictable tumors. The authors present a very well done analysis of the SEER database evaluating predictors of disease specific survival in patients with resected pancreatic neuroendocrine tumors over a twenty year period.
I have three questions:
1. You demonstrate that lymph node involvement is associated with increased risk of disease specific death when controlling for tumor grade, size and patient age. This is an important finding but making the leap from the notion that lymph node positivity is a prognostic variable to a conjecture that a formal lymphadenectomy provides a clinical advantage to all or a select group of patients with NETs is a significant one and one that, as you mention in the discussion, is not fully supported by the data available to you in this study. How should your findings inform our clinical practice?
RESPONSE: The primary purpose of our study was to inform the debate on lymph node involvement and influence on survival in patients with PNETs. Once we establish that nodal involvement is associated with adverse disease specific survival, then the value of lymph node sampling in this group of patients is to provide prognostic information. Formal lymphadenectomy hence optimizes prognostication; whether this translates into a therapeutic benefit is outside the purview of our study and requires further research to establish.
2. There has been a great deal of research done in other GI tumors suggesting lymph node ratio is a better prognostic indicator than lymph node positivity. Can you/have you evaluated lymph node ratio for prognostic power in the SEER NET population?
RESPONSE: The prognostic role of lymph node ratio in PNETs has yet to be defined and indeed should be an area of future study. However, Rindi and colleagues in their 2012 study of over a thousand PNETs (referenced in the manuscript) evaluated the number of positive lymph nodes that correlated best with outcome, and found that one or more positive lymph nodes produced the greatest area under the receiver operator curve (0.67). This was the rationale for our decision to utilize lymph node positivity as a binary variable in our study.
3. The WHO classification/staging system for NETs makes no mention of lymph node involvement. That classification is broken down by histologic grade as assessed by mitotic index or KI-67 staining. How is the classification of grade made in the SEER database? Has the method used in SEER potentially impacted your finding that node positivity was associated with survival independent of tumor grade?
RESPONSE: Per the SEER coding manual, SEER assesses tumor grade on a spectrum related to “how closely the tumor cells resemble the parent tissue (organ of origin).” Grade is classified as well differentiated, moderately differentiated, poorly differentiated or undifferentiated with respect to the organ of origin. As this methodology differs from the more objective measures of Ki-67 or mitotic indices utilized by the WHO stage classification, it is possible that the classification utilized by SEER may lead to different findings as compared to an analysis of the same population using Ki-67 or mitotic indices. However, the European Neuroendocrine Tumor Society staging and grading system put forth in a 2006 edition of Virchow’s Archives by Rindi and colleagues does utilize nodal status as a component of staging while also utilizing Ki-67 index in the establishment of tumor grade. A subsequent validation study by the ENETS group in 2012 demonstrated nodal metastases to be a predictor of survival though it was not specifically evaluated for prognostic value relative independent of grade. Thus, we feel that despite the subjective nature of SEER grade assignment, the finding of decreased survival for those with lymph node metastases is an important one, especially since the WHO classification system is not universally used.
Presented as Plenary Presentation at the 54th Annual Meeting of the Society for Surgery of the Alimentary Tract in conjunction with Digestive Disease Week; May 6, 2014
Rights and permissions
About this article
Cite this article
Curran, T., Pockaj, B.A., Gray, R.J. et al. Importance of Lymph Node Involvement in Pancreatic Neuroendocrine Tumors: Impact on Survival and Implications for Surgical Resection. J Gastrointest Surg 19, 152–160 (2015). https://doi.org/10.1007/s11605-014-2624-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-014-2624-z