Abstract
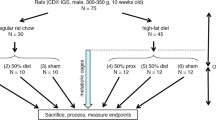
Previous studies have shown that high-fat diet (HFD) enhances adaptation if provided immediately following small bowel resection (SBR). The purpose of this study was to determine if HFD could further enhance villus growth after resection-induced adaptation had already taken place. C57/Bl6 mice underwent a 50 % proximal SBR or sham operation and were then provided a standard rodent liquid diet (LD) ad lib. After a typical period of adaptation (7 days), SBR and sham-operated mice were randomized to receive either LD or HFD (42 % kcal fat) for an additional 7 days. Mice were then harvested, and small intestine was collected for analysis. Adaptation occurred in both SBR groups; however, the SBR/HFD had significantly increased villus height compared to SBR/LD. Reverse transcription–polymerase chain reaction of villus enterocytes showed a marked increase in CD36 expression in the SBR/HFD group compared with SBR/LD mice. While exposure to increased enteral fat alone did not affect villus morphology in sham-operated mice, HFD significantly increased villus growth in the setting of resection-induced adaptation, supporting the clinical utility of enteral fat in augmenting adaptation. Increased expression of CD36 suggests a possible mechanistic role in dietary fat metabolism and villus growth in the setting of short gut syndrome.







Similar content being viewed by others
References
Messing B, Crenn P, Beau P, et al. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology 1999; 117:1043–1050
Squires RH, Duggan C, Teitelbaum DH, et al: Natural history of pediatric intestinal failure: initial report from the Pediatric Intestinal Failure Consortium. J Pediatr 2012; 161:723–728
Menge H, Grafe M, Lorenz-Meyer H, et al: The influence of food intake on the development of structural and functional adaptation following ileal resection in the rat. Gut 1975; 16:468–472
Buts JP, Morin CL,Ling V: Influence of dietary components on intestinal adaptation after small bowel resection in rats. Clin Invest Med 1979; 2:59–66
Dodge ME, Bertolo RF,Brunton JA: Enteral feeding induces early intestinal adaptation in a parenterally fed neonatal piglet model of short bowel syndrome. JPEN J Parenter Enteral Nutr 2012; 36:205–212
Tappenden KA: Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology 2006; 130:S93-S99
Sukhotnik I, Mor-Vaknin N, Drongowski RA, et al: Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int 2004; 20:419–424
Kollman KA, Lien EL,Vanderhoof JA: Dietary lipids influence intestinal adaptation after massive bowel resection. J Pediatr Gastroenterol Nutr 1999; 28:41–45
Vanderhoof JA, Park JH, Herrington MK, et al: Effects of dietary menhaden oil on mucosal adaptation after small bowel resection in rats. Gastroenterology 1994; 106:94–99
Helmrath MA, VanderKolk WE, Can G, et al: Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg 1996; 183:441–449
Guo J, Longshore S, Nair R, et al: Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem 2009; 284:134–140
Sukhotnik I, Gork AS, Chen M, et al: Effect of low fat diet on lipid absorption and fatty-acid transport following bowel resection. Pediatr Surg Int 2001; 17:259–264
Sukhotnik I, Shiloni E, Krausz MM, et al: Low-fat diet impairs postresection intestinal adaptation in a rat model of short bowel syndrome. J Pediatr Surg 2003; 38:1182–1187
Sukhotnik I, Mor-Vaknin N, Drongowski RA, et al: Effect of dietary fat on fat absorption and concomitant plasma and tissue fat composition in a rat model of short bowel syndrome. Pediatr Surg Int 2004; 20:185–191
Hernandez Vallejo SJ, Alqub M, Luquet S, et al: Short-term adaptation of postprandial lipoprotein secretion and intestinal gene expression to a high-fat diet. Am J Physiol Gastrointest Liver Physiol 2009; 296:G782-G792
Abumrad NA,Davidson NO: Role of the gut in lipid homeostasis. Physiol Rev 2012; 92:1061–1085
Nassir F, Wilson B, Han X, et al: CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem 2007; 282:19493–19501
Lobo MV, Huerta L, Ruiz-Velasco N, et al: Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem 2001; 49:1253–1260
Poirier H, Degrace P, Niot I, et al: Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP). Eur J Biochem 1996; 238:368–373
Nauli AM, Nassir F, Zheng S, et al: CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology 2006; 131:1197–1207
Dur S, Krause K, Pluntke N, et al: Gene structure and expression of the mouse APOBEC-1 complementation factor: multiple transcriptional initiation sites and a spliced variant with a premature stop translation codon. Biochim Biophys Acta 2004; 1680:11–23
Goudriaan JR, Dahlmans VE, Febbraio M, et al: Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem 2002; 239:199–202
Lynes M, Narisawa S, Millan JL, et al: Interactions between CD36 and global intestinal alkaline phosphatase in mouse small intestine and effects of high-fat diet. Am J Physiol Regul Integr Comp Physiol 2011; 301:R1738-R1747
Tran TT, Poirier H, Clement L, et al: Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem 2011; 286:25201–25210
Jeppesen PB, Pertkiewicz M, Messing B, et al: Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012 Dec;143(6):1473–1481.
O’Keefe SJ, Jeppesen PB, Gilroy R et al (2013) Safety and Efficacy of Teduglutide After 52 Weeks of Treatment in Patients With Short Bowel Intestinal Failure. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2012.12.029
Hsieh J, Longuet C, Maida A, et al: Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology 2009; 137:997–1005, 1005
Newberry EP,Davidson NO: Intestinal lipid absorption, GLP-2, and CD36: still more mysteries to moving fat. Gastroenterology 2009; 137:775–778
Rowland KJ, Trivedi S, Lee D, et al: Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology 2011; 141:2166–2175
Martin GR, Wallace LE, Hartmann B, et al: Nutrient-stimulated GLP-2 release and crypt cell proliferation in experimental short bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2005; 288:G431-G438
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DK 059288 (Warner), T32 GM008795 (Choi) P30DK52574—Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine, and the Children’s Surgical Sciences Institute of the St. Louis Children’s Hospital Foundation. Dr. Choi is also supported by The Marion and Van Black Endowed Pediatric Surgical Fellowship.
This paper was presented as a plenary presentation at the 54th Annual Meeting of the Society for Surgery of the Alimentary Tract, May 18–21, 2013.
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Richard Hodin (Boston, Massachusetts): Dr. Choi, congratulations on a nice study and thank you for providing the manuscript for my review in advance of this meeting. The Warner lab has been leading the way in the field of gut adaptation for over two decades, and this study represents another piece of this important puzzle. I think it is particularly important that in this study you have addressed the issue of adaptation at a later time point, after the animals have already gone through the early adaptation phase. After all, in the clinical setting, we are generally presented with these patients well after their initial insult and recovery from their surgery, and the question is what can we do to make their lives better, perhaps even helping to make them TPN-independent?
This study clearly shows that dietary composition is going to be a key component of the gut adaptation response. There are a couple of questions that I have for you:
(1) Even the sham, HFD mice apparently had less body fat. This seems counter-intuitive. Obviously, we think of a high-fat diet as leading to more body fat, and there are certainly many experimental studies that have demonstrated a high-fat diet causing obesity, insulin resistance, fatty liver, etc. Can you explain this apparent discrepancy?
(2) There has been a lot of recent interest in GLP-2, suggesting that it may help patients become less dependent on TPN. Can you tell us whether you have given GLP-2 to your mice and what the impact is relative to dietary manipulations? For example, if you add GLP-2 to the HFD, will you get more adaptation?
Closing Discussant
Dr. Pamela Choi: Thank you, Dr. Hodin for your insightful questions.
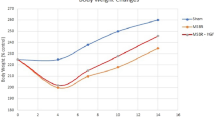
In response to your first question, indeed this finding that even the sham-operated mice on HFD have less body fat is counter-intuitive. However, these mice did also experience a significant decrease in weight loss within the first 7 days after surgery although not as much as resected mice. This suggests that even sham mice may be experiencing some degree of catabolism. Under these circumstances, it is possible that fat may be metabolized in a way to stimulate protein production. Saturated long-chain fatty acids, which are present in high quantities in the HFD, are more calorically dense molecules than monounsaturated or polyunsaturated long-chain fatty acids. This leads to increase in ATP production which may stimulate protein production as part of the recovery phase from the acute stressor.
We did not give exogenous GLP-2 in our experiment to see if there is an additive effect. However, we were indeed interested in GLP-2 as a potential mechanism for high-fat diet-induced adaptation. Other studies have shown that GLP-2 levels are increased after resection and high-fat enteral feeding independently. Additionally, GLP-2, in addition to its intestinotrophic effects, has also been shown to stimulate CD36 expression. Unfortunately, we were unable to measure GLP-2 as we did not collect serum prior to harvest. However, the trophic effects of GLP-2 are carried out through IGF-1 and the IGF-1 receptor, and so repeating this experiment on IGF-1R-floxed mice may prove if GLP-2 is a critical mediator in HFD-induced enhanced adaptation after resection.
Rights and permissions
About this article
Cite this article
Choi, P.M., Sun, R.C., Guo, J. et al. High-Fat Diet Enhances Villus Growth During the Adaptation Response to Massive Proximal Small Bowel Resection. J Gastrointest Surg 18, 286–294 (2014). https://doi.org/10.1007/s11605-013-2338-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-013-2338-7