Abstract
Purpose
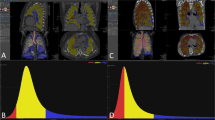
To evaluate the feasibility of automated quantitative analysis with a three-dimensional (3D) computer-aided system (i.e., Gaussian histogram normalized correlation, GHNC) of computed tomography (CT) images from different scanners.
Materials and methods
Each institution’s review board approved the research protocol. Informed patient consent was not required. The participants in this multicenter prospective study were 80 patients (65 men, 15 women) with idiopathic pulmonary fibrosis. Their mean age was 70.6 years. Computed tomography (CT) images were obtained by four different scanners set at different exposures. We measured the extent of fibrosis using GHNC, and used Pearson’s correlation analysis, Bland–Altman plots, and kappa analysis to directly compare the GHNC results with manual scoring by radiologists. Multiple linear regression analysis was performed to determine the association between the CT data and forced vital capacity (FVC).
Results
For each scanner, the extent of fibrosis as determined by GHNC was significantly correlated with the radiologists’ score. In multivariate analysis, the extent of fibrosis as determined by GHNC was significantly correlated with FVC (p < 0.001). There was no significant difference between the results obtained using different CT scanners.
Conclusion
Gaussian histogram normalized correlation was feasible, irrespective of the type of CT scanner used.




Similar content being viewed by others
References
Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824.
Azuma A, Nukiwa T, Tsuboi E, Suga M, Abe S, Nakata K, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–7.
Taniguchi H, Ebina M, Kondoh Y, Ogura T, Azuma A, Suga M, Pirfenidone Clinical Study Group in Japan, et al. Pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–9.
Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabe U, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–82.
King TE Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, ASCEND Study Group, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–92.
Noble PW, Albera C, Bradford WZ, Costabel U, Glassberg MK, Kardatzke D, CAPACITY Study Group, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–9.
Lynch DA, Travis WD, Müller NL, Galvin JR, Hansell DM, Grenier PA, et al. Idiopathic interstitial pneumonias: CT features. Radiology. 2005;236:10–21.
Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–7.
Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177:433–9.
Maldonado F, Moua T, Rajagopalan S, Karwoski RA, Raghunath S, Decker PA, et al. Automated quantification of radiological patterns predicts survival in idiopathic pulmonary fibrosis. Eur Respir J. 2014;43:204–12.
Ley B, Elicker BM, Hartman TE, Ryerson CJ, Vittinghoff E, Ryu JH, et al. Idiopathic pulmonary fibrosis: CT and risk of death. Radiology. 2014;273:570–9.
Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, Bowler RP, COPDGene CT Workshop Group, et al. A combined pulmonary–radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. COPD. 2012;9:151–9.
Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–44.
Park SO, Seo JB, Kim N, Park SH, Lee YK, Park BW, et al. Feasibility of automated quantification of regional disease patterns depicted on high-resolution computed tomography in patients with various diffuse lung diseases. Korean J Radiol. 2009;10:455–63.
Hoffman EA, Reinhardt JM, Sonka M, Simon BA, Guo J, Saba O, et al. Characterization of the interstitial lung diseases via density-based and texture-based analysis of computed tomography images of lung structure and function. Acad Radiol. 2003;10:1104–18.
Korfiatis PD, Karahaliou AN, Kazantzi AD, Kalogeropoulou C, Costaridou LI. Texture-based identification and characterization of interstitial pneumonia patterns in lung multidetector CT. IEEE Trans Inf Technol Biomed. 2010;14:675–80.
Uppaluri R, Hoffman EA, Sonka M, Hunninghake GW, McLennan G. Interstitial lung disease: a quantitative study using the adaptive multiple feature method. Am J Respir Crit Care Med. 1999;159:519–25.
Zavaletta VA, Bartholmai BJ, Robb RA. High resolution multidetector CT-aided tissue analysis and quantification of lung fibrosis. Acad Radiol. 2007;14:772–87.
Rosas IO, Yao J, Avila NA, Chow CK, Gahl WA, Gochuico BR. Automated quantification of high-resolution CT scan findings in individuals at risk for pulmonary fibrosis. Chest. 2011;140:1590–7.
Wang J, Li F, Li Q. Automated segmentation of lungs with severe interstitial lung disease in CT. Med Phys. 2009;36:4592–9.
Kauczor HU, Heitmann K, Heussel CP, Marwede D, Uthmann T, Thelen M. Automatic detection and quantification of ground-glass opacities on high-resolution CT using multiple neural networks: comparison with a density mask. AJR Am J Roentgenol. 2000;175:1329–34.
Yoon RG, Seo JB, Kim N, Lee HJ, Lee SM, Lee YK, et al. Quantitative assessment of change in regional disease patterns on serial HRCT of fibrotic interstitial pneumonia with texture-based automated quantification system. Eur Radiol. 2013;23:692–701.
Sluimer IC, Prokop M, Hartmann I, van Ginneken B. Automated classification of hyperlucency, fibrosis, ground glass, solid, and focal lesions in high-resolution CT of the lung. Med Phys. 2006;33:2610–20.
Raghunath S, Rajagopalan S, Karwoski RA, Maldonado F, Peikert T, Moua T, et al. Quantitative stratification of diffuse parenchymal lung diseases. PLoS One. 2014;9:e93229.
Asakura A, Gotoh T, Iwasawa T, Saito K, Akasaka H. Classification system of the CT images with nonspecific interstitial pneumonia. J Inst Image Electron Eng Jpn. 2004;33:180–8.
Iwao Y, Gotoh T, Kagei S, Iwasawa T, Tsuzuki Mde S. Integrated lung field segmentation of injured region with anatomical structure analysis by failure–recovery algorithm from chest CT images. Biomed Signal Process Control. 2014;12:28–38.
Iwasawa T, Asakura A, Sakai F, Kanauchi T, Gotoh T, Ogura T, et al. Assessment of prognosis of patients with idiopathic pulmonary fibrosis by computer-aided analysis of CT images. J Thorac Imaging. 2009;24:216–22.
Iwasawa T, Ogura T, Sakai F, Kanauchi T, Komagata T, Baba T, et al. CT analysis of the effect of pirfenidone in patients with idiopathic pulmonary fibrosis. Eur J Radiol. 2014;83:32–8.
Japanese Respiratory Society. Guideline for pulmonary function test. 1st ed. Tokyo: Medical View; 2004.
Best AC, Lynch AM, Bozic CM, Miller D, Grunwald GK, Lynch DA. Quantitative CT indexes in idiopathic pulmonary fibrosis: relationship with physiologic impairment. Radiology. 2003;228:407–14.
Lynch DA, Godwin JD, Safrin S, Starko KM, Hormel P, Brown KK, et al. Idiopathic Pulmonary Fibrosis Study Group. High-resolution computed tomography in idiopathic pulmonary fibrosis: diagnosis and prognosis. Am J Respir Crit Care Med. 2005;172:488–93.
Sluimer I, Schilham A, Prokop M, van Ginneken B. Computer analysis of computed tomography scans of the lung: a survey. IEEE Trans Med Imaging. 2006;25:385–405.
Katzenstein AL. Idiopathic interstitial pneumonia. In: Katzenstein AL, editor. Katzenstein and Askin’s surgical pathology of non-neoplastic lung disease. 4th ed. Philadelphia: Saunders Elsevier; 2006. p. 51–84.
Iwasawa T, Iwao Y, Goto T, Asakura A, Komagata T, Ogura T, et al. Computer-aided segmentation system for 3D chest CT. Rinsho Hoshasen. 2012;57:41–7 (in Japanese).
Yamaguchi K, Mori M, Kawai A, Takasugi T, Oyamada Y, Koda E. Inhomogeneities of ventilation and the diffusing capacity to perfusion in various chronic lung diseases. Am J Respir Crit Care Med. 1997;156:86–93.
Acknowledgments
This study was approved by each institutional review board (IRB number, Kanagawa Cardiovascular & Respiratory Center 23-1, Saitama Cardiovascular and Respiratory Center 2011004).
This study was supported by the Kanagawa Cancer Research Fund, in part supported by a joint project from JSPS/CAPES under the Japan–Brazil Research Cooperative Program and Grants-in-Aid for Scientific Research (24500539), and in part supported by JSPS KAKENHI grant number 15K09901.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Appendix
Appendix
The Gaussian histogram normalized correlation (GHNC) method classifies pixels in a target image into different categories. In the GHNC method, a set of Gaussian histograms of CT attenuation values of the original image and the differential image are extracted from samples of each category in advance. The normalized correlations between the Gaussian histograms in the surrounding local 50-pixel area of a pixel and those of the categories are then compared for classification into the predefined categories. To avoid the effects of random noise, pixel attenuation is assumed to have random noise with a Gaussian distribution. Gaussian convolution filtering is applied to the extracted histograms from the local area and the samples in the categories.
The Gaussian histogram of the predesigned sample is obtained from the following formula:
in which α is the Hounsfield units of each pixel and N α is the number of pixels in the predesigned sample area D α. The variable σ is the standard deviation of the Gaussian random noise.
The Gaussian histogram of the target area D β is given by
in which β and N β also denote the Hounsfield units and the number of pixels in the target area D β, respectively.
The normalized correlation between α g(x) and β g(x) is given by the formula
In our method, the normalized correlations are calculated in the local area of each target pixel for the original and differential images. The product of both correlations is then used as the similarity of the target and the predesigned sample pattern in each category to classify the target pixels into the predesigned categories.
About this article
Cite this article
Iwasawa, T., Kanauchi, T., Hoshi, T. et al. Multicenter study of quantitative computed tomography analysis using a computer-aided three-dimensional system in patients with idiopathic pulmonary fibrosis. Jpn J Radiol 34, 16–27 (2016). https://doi.org/10.1007/s11604-015-0496-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-015-0496-0