Abstract
Objective
The aim of this study was to explore the effect of norepinephrine (NE) on renal cortical and medullary blood flow in atherosclerotic rabbits without renal artery stenosis.
Methods
Atherosclerosis was induced in 21 New Zealand white rabbits by feeding them a cholesterol-rich diet for 16 weeks. Thirteen healthy New Zealand white rabbits were randomly selected as controls. After atherosclerosis induction, standard ultrasonography was performed to confirm that there was no plaque or accelerated flow at the origin of the renal artery. Contrast-enhanced ultrasound (CEUS) was performed at baseline and during intravenous injection of NE. The degree of contrast enhancement of renal cortex and medulla after the injection of contrast agents was quantified by calculating the enhanced intensity.
Results
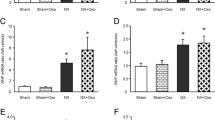
The serum nitric oxide (NO) level in atherosclerotic rabbits was higher than that in healthy rabbits (299.6±152 vs. 136.5±49.5, P<0.001). The infusion of NE induced a significant increase in the systolic blood pressure (112±14 mmHg vs. 84±9 mmHg, P=0.016) and a significant decrease in the enhanced intensity in renal cortex (17.78±2.07 dB vs. 21.19±2.03 dB, P<0.001) and renal medulla (14.87±1.82 dB vs. 17.14±1.89 dB, P<0.001) during CEUS. However, the enhanced intensity in the cortex and medulla of healthy rabbits after NE infusion showed no significant difference from that at baseline.
Conclusion
NE may reduce renal cortical and medullary blood flow in atherosclerotic rabbits without renal artery stenosis, partly by reducing the serum NO level.
Similar content being viewed by others
References
Motiejunaite J, Amar L, Vidal-Petiot E. Adrenergic receptors and cardiovascular effects of catecholamines. Ann Endocrinol (Paris), 2021,82(3–4):193–197
Profumo E, Buttari B, D’Arcangelo D, et al. The Nutraceutical Dehydrozingerone and Its Dimer Counteract Inflammation- and Oxidative Stress-Induced Dysfunction of In Vitro Cultured Human Endothelial Cells: A Novel Perspective for the Prevention and Therapy of Atherosclerosis. Oxid Med Cell Longev, 2016,2016:1246485
Berkenboom G, Depierreux M, Fontaine J. The influence of atherosclerosis on the mechanical responses of human isolated coronary arteries to substance P, isoprenaline and noradrenaline. Br J Pharmacol, 1987,92(1):113–120
Wroblewski T, Witanowska A. The contractile response of the normal and atherosclerotic aortic strips to noradrenaline, and potassium ions. Acta Physiol Pol, 1982,33(4):353–360
Li T, Mao Y, Zhao B, et al. Value of contrast-enhanced ultrasound for diagnosis and follow-up of renal artery stenosis in patients with chronic kidney disease. Abdom Radiol (NY), 2022,47(5):1853–1861
Wang L, Mohan C. Contrast-enhanced ultrasound: A promising method for renal microvascular perfusion evaluation. J Transl Int Med, 2016,4(3):104–108
Sun J, Liu K, Tang QY, et al. Correlation between enhanced intensity of atherosclerotic plaque at contrast-enhanced ultrasonography and density of histological neovascularization. J Huazhong Univ Sci Technolog Med Sci, 2013,33(3):443–446
Sun J, Deng YB, Liu K, et al. Effects of noradrenaline and adenosine triphosphate on the degree on contrast enhancement in a rabbit model of atherosclerosis during contrast-enhanced ultrasonography. Ultrasound Med Biol, 2014,40(11):2655–2661
Li Y, Chahal N, Senior R, et al. Reproducible Computer-Assisted Quantification of Myocardial Perfusion with Contrast-Enhanced Ultrasound. Ultrasound Med Biol, 2017,43(10):2235–2246
Juárez-Orozco LE, Szymanski MK, Hillege HL, et al. Imaging of cardiac and renal perfusion in a rat model with 13N-NH 3 micro-PET. Int J Cardiovasc Imaging, 2015,31(1):213–219
Schumann J, Henrich EC, Strobl H. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst Rev, 2018,1(1):CD009669
Schneider AG, Goodwin MD, Schelleman A, et al. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: a pilot study. Crit Care, 2014,18(6):653
Burke M, Pabbidi MR, Farley J, et al. Molecular mechanisms of renal blood flow autoregulation. Curr Vasc Pharmacol, 2014,12(6):845–858
Anderson WP, Korner PI, Selig SE. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J Physiol, 1981,321:21–30
Ferreira MG, Fonteles MC. Different classes of PAF-antagonists block norepinephrine-induced vascular escape and tachyphylaxis in the isolated rabbit kidney. Res Commun Mol Pathol Pharmacol, 1996,94(2):147–155
Moss NG, Kopple TE, Arendshorst WJ. Renal vasoconstriction by vasopressin V1a receptors is modulated by nitric oxide, prostanoids, and superoxide but not the ADP ribosyl cyclase CD38. Am J Physiol Renal Physiol, 2014,306(10):F1143–1154
Wroblewski TE, Grojec M. Vasomotor escape from noradrenaline in the renal, mesenteric and hindquarter vascular beds in spontaneously hypertensive rats (SHR). Acta Physiol Pol, 1984,35(4):324–329
Chen JY, Ye ZX, Wang XF, et al. Nitric oxide bioavailability dysfunction involves in atherosclerosis. Biomed Pharmacother, 2018,97:423–428
Arger PH, Sehgal CM, Pugh CR, et al. Evaluation of change in blood flow by contrast-enhanced power Doppler imaging during norepinephrine-induced renal vasoconstriction. J Ultrasound Med, 1999,18(12):843–851
Evans RG, Madden AC, Denton KM. Diversity of responses of renal cortical and medullary blood flow to vasoconstrictors in conscious rabbits. Acta Physiol Scand, 2000,169(4):297–308
Correia AG, Madden AC, Bergström G, et al. Effects of renal medullary and intravenous norepinephrine on renal antihypertensive function. Hypertension, 2000,35(4):965–970
Mukai K, Kuda Y, Shibamoto T, et al. Renal response to anaphylaxis in anesthetized rats and isolated perfused rat kidneys: roles of nitric oxide. J Physiol Sci, 2018,68(5):689–697
Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol, 2006,70(5):1469–1480
LeDoux D, Astiz ME, Carpati CM, et al. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med, 2000,28(8):2729–2732
Lenz, T. Ischemic renal disease and renal artery stenosis. Internist (Berl), 2015,56(3):248–254
Schoepe R, McQuillan S, Valsan D, et al. Atherosclerotic Renal Artery Stenosis. Adv Exp Med Biol, 2017,956:209–213
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that there is no conflict of interest with any financial organization or corporation or individual that can inappropriately influence this work.
Additional information
This work was supported by grants from Health and Family Planning Commission Foundation of Hubei Province (No. WJ2017M080) and the National Natural Science Foundation of China (No. 81601507).
Rights and permissions
About this article
Cite this article
Wang, Jy., Sun, J., Deng, Yb. et al. Effects of Norepinephrine on Renal Cortical and Medullary Blood Flow in Atherosclerotic Rabbits. CURR MED SCI 42, 1172–1177 (2022). https://doi.org/10.1007/s11596-022-2626-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2626-0