Abstract
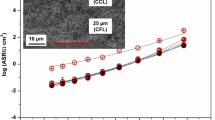
As for the commonly studied La0.6Sr0.4Co0.2Fe0.8O3-δ (6428), here, a very low area-specific resistance (ASR) was measured for La0.6Sr0.4Co0.8Fe0.2O3-δ (6482) cathode deposited on a Ce0.9Gd0.1O2-δ (GDC) electrolyte with addition of a thin (1 μm) dense LSCF film deposited by spin coating at the interface between the GDC electrolyte and a 40-μm-thick screen-printed electrode. The ASR ranged from 1 Ω.cm2 at 500 °C, 0.11 Ω.cm2 at 625 °C and value as low as 0.03 Ω.cm2 at 700 °C. Impedance spectra collected in between 500 and 700 °C were carefully studied. They could all be modelled with two R//CPE in series which are likely associated to the oxygen reduction reaction itself (dissociation/adsorption/ionization) at low frequency and to the oxide ion transfer at the electrode/electrolyte interface at high frequency.













Similar content being viewed by others
References
Li W, Lu K, Xia Z (2013) J Power Sources 237:119–127
Steele BCH, Bae JM (1998) Solid State Ionics 106:255–261
Lane JA, Benson SJ, Waller D, Kilner JA (1999) Solid State Ionics 121:201–208
Hwang HJ, Moon JW, Lee S, Lee EA (2005) J Power Sources 145:243–248
Liu M, Ding D, Blinn K, Li X, Nie L, Liu M (2012) Int J Hydrog Energy 37:8613–8620
Marinha D, Dessemond L, Scott Cronin J, Wilson JR, Barnett SA, Djurado E (2011) Chem Mater 23:5340–5348
Marinha D, Dessemond L, Djurado E (2011) ECS. Transactions 35(1):2283–2294
Marinha D, Hayd J, Dessemond L, Ivers-Tiffée E, Djurado E (2011) J Power Sources 196:5084–5090
Hildenbrand N, Boukamp BA, Nammensma P, Blank DHA (2011) Solid State Ionics 192:12–15
Hildenbrand N, Nammensma P, Blank DHA, Bouwmeester HJM, Boukamp BA (2013) J Power Sources 238:442–453
Berenov AV, Atkinson A, Kilner JA, Bucher E, Sitte W (2010) Solid State Ionics 181:819–826
Hayd J, Yokokawa H, Ivers-Tiffée E (2013) J Electrochem Soc 160:F351–F359
Yamamoto O (2000) Electrochim Acta 45:2423–2435
Kordesch K, Simader G (1996) Fuel cells and their applications. VCH, Weinheim/Wiley, NY (U.S.A)
Ullman H, Trofimenko N, Tietz F, Stöver D, Ahmad-Khanlou A (2000) Solid State Ionics 138:79–90
Boukamp BA (1995) J Electrochem Soc 142:1885–1894
ZView software, version 3.4a, Scribner Associates Inc., Southern Pines (N. C., USA)
Cole KS, Cole RH (1941) J Chem Phys 9:341–351
Boukamp BA (2004) Solid State Ionics 169:65–73
Orazem ME, Pébère N, Tribollet P (2006) J Electrochem Soc 153:B129–B136
Barsoukov E, Ross MacDonald J (eds) (2005) Impedance spectroscopy: theory, experiment and applications. Wiley-Interscience, Hoboken (N. J., U.S.A)
Ibusuki Y, Kunigo H, Hirata Y, Sameshima S, Matsunaga N (2011) J Eur Ceram Soc 31(14):2663–2669
Lai W, Haile SM (2005) J Am Ceram Soc 88:2979–2997
Nielsen J, Jacobsen T, Wandel M (2011) Electrochim Acta 56:7963–7974
Boukamp BA (1986) Solid State Ionics 20:31–44
Baumann FS, Fleig J, Habermeier H-U, Maier J (2006) Solid. State Ionics 177(11–12):1071–1081
K. Dumaisnil, Ph. D thesis University of Littoral-Côte d’Opale (2015) Calais (France)
Adler SB (2004) Chem Rev 104:4791–4843
Simrick NJ, Bieberle-Hütter A, Ryll TM, Kilner JA, Atkinson A, Rupp JLM (2012) Solid State Ionics 206:7–16
Leonide A, Sonn V, Weber A, Ivers-Tiffée E (2008) J Electrochem Soc 155:B36–B41
Baumann FS, Fleig J, Habermeier H-U, Maier J (2006) Solid State Ionics 177(35–36):3187–3191
Zoltowski P (1998) J Electroanal Chem 443:149–154
Acknowledgments
The authors are grateful to the Région Nord-Pas de Calais for the attribution of an “Emergent Project” called OPERAH which funded a part of this work.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The complex impedance of a resistance R in parallel with a constant phase element CPE circuit can easily be calculated and the determination of the characteristic parameters such as F C, R, α, Q and C can be made.
For the definition of the CPE, we took the one proposed by P. Zoltowski [32] as follows:
α and Q are constants independent of frequency but which could depend on temperature. Moreover, Q is directly proportional to the active area.
The complex impedance of the R//CPE circuit can be written as follows:
so,
and
From Nyquist diagram, it can be seen that Z” presents a maximum Z”C at a characteristic frequency F C . The expression of F C is obtained from the derivative dZ”/dF = 0. After some calculations, we obtain the following:
Then, it is possible to determine the expressions of Z’C and Z”C at this characteristic frequency F C :
It can be noted that Eqs. 2, 3 and 4can be written as a function of R, α, F and F C by replacing RQω α by (F/F C)α and (RQω α)2 by (F/F C)2α.
Now, it is possible to determine the values of R, α and F C with graphical means as follows:
-
R is obtained from Z’(logF) curve such as R = R 0 − R ∝ where R 0 corresponds to the Z’ value measured at the lowest frequency and R ∝ to the highest frequency. In the same way, R can be obtained from the Nyquist diagram with the intercepts of the curve with the Z’ axis.
-
F C is obtained from the maximum Z” C of the peak of Z”(logF) curve. It can also be obtained from the Nyquist diagram as F C corresponds to the frequency of the maximum Z” C .
-
α is obtained from the amplitude Z” C such as α \( =-\frac{4}{\pi}\ {tan}^{-1}\left(\frac{2{Z^{"}}_{\mathrm{C}}}{R}\right) \). It can also be obtained from the Nyquist diagram such as \( \alpha =\frac{\beta \left(\mathrm{rad}\right)}{\pi} \) or \( \alpha =\frac{\beta \left(\mathrm{degrees}\right)}{180} \), where β is the angle determined by the center of the semicircle and the two radius joining respectively R 0 and R ∝ on the Z’ axis. In this case the use of a compass and a protractor permits to determine α.
So, for each of the three parameters R, α and F C two values can be determined graphically by two manners. Then, for each parameter, a mean value can be calculated which also permits to deduce a mean value for the parameter Q as follows:
It is possible to introduce a capacitance C of a parallel R//C circuit which would have the same characteristic frequency F C as the R//CPE circuit, i.e.:
C is not the physical capacitance of the sample but an equivalent capacitance as the R//C circuit simulates only one time constant τ c = RC whereas in the sample at high temperature, there are a distribution of time constants.
Rights and permissions
About this article
Cite this article
Dumaisnil, K., Carru, JC., Fasquelle, D. et al. Promising performances for a La0.6Sr0.4Co0.8Fe0.2O3-δ cathode with a dense interfacial layer at the electrode-electrolyte interface. Ionics 23, 2125–2132 (2017). https://doi.org/10.1007/s11581-017-2061-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2061-6