Abstract
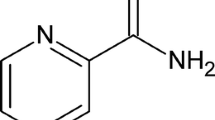
In the work accounted here, the electro-oxidation of one of the pyrimidine bases thymine is studied on a glassy carbon electrode modified with the biopolymer, poly (l-glutamic acid), as the probe. On the probe, the overpotential for thymine oxidation in alkaline medium has been significantly decreased attesting the electrocatalytic nature of the biopolymer film. The experimental parameters to obtain the lowest oxidation potential have been optimised and the probe calibrated for the determination of thymine. In the square wave mode, using 0.1 M NaOH as the supporting electrolyte, the oxidation peak current was found to be linear in the range from 30 to 1000 μM, with a detection limit of 9.2 μM. The diffusion coefficient of thymine in 0.1 M sodium hydroxide has been determined using chronoamperometric studies. To study the mechanistic aspects of the electro-oxidation process, variation of the oxidation peak parameters with scan rate has been studied in the linear sweep mode. Electrochemical kinetic parameters, namely charge transfer coefficient α and the standard heterogeneous rate constant k s for the electro-oxidation, have also been determined. The determination of thymine in spiked synthetic blood serum and urine has been conducted to demonstrate the application of the sensor for thymine determination in real samples.

Electrocatalytic oxidation of thymine on the biopolymer [poly(glutamic acid)]-modified glassy carbon electrode.









Similar content being viewed by others
References
Weaver RF (2012) Molecular biology. McGraw-Hill, New York
Bhagwat AS, Lieb M (1998) Bacterial genomes. In: de Bruijn FJ, Lupski JR, Weinstock GM (eds) Mechanism of avoidance of 5-methylcytosine to thymine mutations in bacteria. Springer US, Boston, pp 119–129
Brett AMO, Matysik FM (1997) Voltammetric and sonovoltammetric studies on the oxidation of thymine and cytosine at a glassy carbon electrode. J Electroanal Chem 429:95–99
Arvand M, Mazhabi RM, Niazi A (2013) Simultaneous determination of guanine, adenine and thymine using a modified carbon paste electrode by TiO2 nanoparticles-magnesium(II) doped natrolite zeolite. Electrochim Acta 89:669–679
Sun W, Xi M, Zhang L, Zhan T, Gao H, Jiao K (2010) Electrochemical behaviors of thymine on a new ionic liquid modified carbon electrode and its detection. Electrochim Acta 56:222–226
Han H, Li J, Li Y, Pang X (2013) Electrochemical methods for determination of thymine in medical pipefish samples based on HPMαFP/Ppy/GCE-modified electrode. Ionics 19:989–996
Anu Prathap MU, Srivastava R, Satpati B (2013) Simultaneous detection of guanine, adenine, thymine, and cytosine at polyaniline/MnO2 modified electrode. Electrochim Acta 114:285–295
Devadas B, Rajkumar M, Chen S (2013) Simultaneous determination of adenine and thymine in presence of guanine at electrochemically activated glassy carbon electrode. Int J Electrochem Sci 8:5241–5249
Deng C, Xia Y, Xiao C, Nie Z, Yang M, Si S (2012) Electrochemical oxidation of purine and pyrimidine bases based on the boron-doped nanotubes modified electrode. Biosens Bioelectron 31:469–474
Feng L, Zhang X, Liu P, Xiong H, Wang S (2011) An electrochemical sensor based on single-stranded DNA–poly(sulfosalicylic acid) composite film for simultaneous determination of adenine, guanine, and thymine. Anal Biochem 419:71–75
Shen Q, Wang X (2009) Simultaneous determination of adenine, guanine and thymine based on β-cyclodextrin/MWNTs modified electrode. J Electroanal Chem 632:149–153
Tang C, Yogeswaran U, Chen S (2009) Simultaneous determination of adenine guanine and thymine at multi-walled carbon nanotubes incorporated with poly(new fuchsin) composite film. Anal Chim Acta 636:19–27
Brett AMO, Piedade JAP, Silva LA, Diculescu VC (2004) Voltammetric determination of all DNA nucleotides. Anal Biochem 332:321–329
Thomas D, Rasheed Z, Jagan JS, Girish Kumar K (2015) Study of kinetic parameters and development of a voltammetric sensor for the determination of butylated hydroxyanisole (BHA) in oil samples. J Food Sci Technol 52:6719–6726
Thomas D, Vikraman AE, Jos T, Girish Kumar K (2015) Kinetic approach in the development of a gold nanoparticle basedvoltammetric sensor for Sudan I. LWT Food Sci Technol 63:1294–1300
Vikraman AE, Thomas D, Cyriac ST, Girish Kumar K (2014) Kinetic and thermodynamic approach in the development of a voltammetric sensor for sunset yellow. J Electrochem Soc 161:B305–B311
Li R, Ma B, Wu S, Zhao J, Lei X (2008) Electron transfer in electroactive poly(glutamic acid). Eur Polym J 44:2231–2235
Gu Y, Liu W, Chen R, Zhang L, Zhang Z (2013) β-Cyclodextrin-functionalized gold nanoparticles/poly(L-cysteine) modified glassy carbon electrode for sensitive determination of metronidazole. Electroanal 25:1209–1216
Anuja EV (2015) Novel electrochemical and fluorescence sensors for food additives and neurotransmitters. Dissertation, Cochin University of Science and Technology.
Brownson DAC, Banks CE (2014) The handbook of graphene electrochemistry. Springer London, London
Yu A, Chen H (1999) Electrocatalytic oxidation and determination of ascorbic acid at poly(glutamic acid) chemically modified electrode. Anal Chim Acta 344:181–185
Jesny S, Menon S, Girish Kumar K (2016) Simultaneous determination of guanine and adenine in the presence of uric acid by poly (para toluene sulfonic acid) mediated electrochemical sensor in alkaline medium. RSC Adv 6:75741–75748
Vikraman AE, Rasheed Z, Rajith L, Lonappan LA, Girish Kumar K (2013) MWCNT-modified gold electrode sensor for the determination of propyl gallate in vegetable oils. Food Anal Methods 6:775–780
Laviron E, Roullier L, Degrand C (1980) A multilayer model for the study of space distributed redox modified electrodes: part II. Theory and application of linear potential sweep voltammetry for a simple reaction. J Electroanal Chem 112:11–23
Acknowledgements
The authors, K. Girish Kumar and S. Jesny, acknowledge the University Grants Commission (UGC), Government of India, for providing monetary aid in the form of One-Time Research Grant and Teacher Fellowship under the Faculty Development Programme, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Jesny, S., Rasheed, Z. & Girish Kumar, K. A biopolymer-based voltammetric sensor for thymine: Elucidation of electrochemical kinetics. Ionics 23, 1533–1540 (2017). https://doi.org/10.1007/s11581-016-1958-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1958-9