Abstract
Tuberculosis (TB) and human immunodeficiency virus (HIV) are the two major public health emergencies in the Philippines. The country is ranked fourth worldwide in TB incidence cases despite national efforts and initiatives to mitigate the disease. Concurrently, the Philippines has the fastest-growing HIV epidemic in Asia and the Pacific region. The TB-HIV dual epidemic forms a lethal combination enhancing each other’s progress, driving the deterioration of immune responses. In order to understand and describe the transmission dynamics and epidemiological patterns of the co-infection, a compartmental model for TB-HIV is developed. A class of people living with HIV (PLHIV) who did not know their HIV status is incorporated into the model. These unaware PLHIV who do not seek medical treatment are potential sources of new HIV infections that could significantly influence the disease transmission dynamics. Sensitivity analysis using the partial rank correlation coefficient is performed to assess model parameters that are influential to the output of interests. The model is calibrated using available Philippine data on TB, HIV, and TB-HIV. Parameters that are identified include TB and HIV transmission rates, progression rates from exposed to active TB, and from TB-latent with HIV to active infectious TB with HIV in the AIDS stage. Uncertainty analysis is performed to identify the degree of accuracy of the estimates. Simulations predict an alarming increase of 180% and 194% in new HIV and TB-HIV infections in 2025, respectively, relative to 2019 data. These projections underscore an ongoing health crisis in the Philippines that calls for a combined and collective effort by the government and the public to take action against the lethal combination of TB and HIV.
Similar content being viewed by others
1 Introduction
Tuberculosis (TB) is an infectious disease caused by the bacillus Mycobacterium tuberculosis (M. tb) which typically affects the lungs (pulmonary TB) but can also affect other organs (extrapulmonary TB) including kidneys, bones, and liver. The disease is spread from person to person through airborne transmission route, in particular, when an individual sick with TB expel the M. tb in the air by coughing. Approximately, a quarter of the world’s population (equivalent to 2 billion people) is infected with M. tb, of which, 5–10% will develop TB disease during their lifetime. However, a higher risk of developing TB disease has been observed to those with compromised immune systems and those affected by risk factors including malnutrition, diabetes, smoking, and alcohol consumption. In 2020, it has been estimated that 10 million people fell ill with TB and about 1.5 million died worldwide. TB is considered the second leading infectious killer after COVID-19, albeit curable and preventable (World Health Organization 2021, 2022a).
The human immunodeficiency virus (HIV), on the other hand, is a virus which targets the individual’s immune system and weakens people’s defense systems against many infections and some types of cancers. HIV infection can be transmitted through the exchange of body fluids such as blood, semen, vaginal fluid, and breast milk. The virus destroys CD4+ cells, commonly called T-cells, which help the immune system fight off infections. Acquired immunodeficiency syndrome (AIDS), the most severe phase of HIV infection, may take several years to develop. In 2020, an estimate of 1.5 million people acquired HIV and about 680,000 people died from HIV-related causes (CDC 2018; World Health Organization 2022b).
TB and HIV are interconnected in various unknown pathways compromising the host’s immune system. These two diseases form a lethal combination, aggravating each other’s progress, leading to deterioration of immunological functions (Bruchfeld et al. 2015; Diedrich and Flynn 2011; Getahun et al. 2010; Abdool Karim et al. 2010; Kwan and Ernst 2011; Modjarrad and Vermund 2010; Pawlowski et al. 2012). People living with HIV (PLHIV), i.e., individuals infected with HIV, are 18 times more likely to develop TB than those without HIV (World Health Organization 2021, 2022a). HIV increases the risk of activation of latent TB because of the decrease in immunity (WHO Europe 2006). Additionally, HIV fuels the progression rate of recurrent TB which is one of the reasons of multi-drug resistant TB infections (Chaisson and Churchyard 2010; Mesfin et al. 2014). Infection with M. tb negatively affects the immune response to HIV, intensifying the progression from HIV infection to AIDS (Bruchfeld et al. 2015; Pawlowski et al. 2012). Approximately 215,000 people died of HIV-associated TB in 2020. The synergistic interactions between TB and HIV pose a staggering health concern worldwide, particularly in resource-limited regions such as African and Asian countries (World Health Organization 2022a).
Worldwide, the Philippines is ranked fourth in terms of the 2020 number of TB incident cases (World Health Organization 2021). It has been reported that about a million Filipinos have active TB, the third highest prevalence rate after South Africa and Lesotho. Though TB is highly curable, about 70 Filipinos die every day from this disease (Department of Health 2019; WHO 2019). For the past 15 years, TB incidence in the Philippines is almost stagnant, despite national efforts and several initiatives launched to end this disease (Department of Health 2019, 2020). Evidently, TB remains to be a major public health threat in the country. Along with TB, HIV crisis in the Philippines is a health emergency (Gangcuangco 2019). With a 237% increase in annual HIV infections from 2010 to 2020, the Philippines has the fastest growing HIV epidemic in Asia and the Pacific region. During the same period, the reported AIDS-related deaths have increased by 315%. In 2020, the total estimated number of PLHIV hit 115,100 (Department of Health - Epidemiology Bureau 2020; UNAIDS 2022). Furthermore, the number of TB-HIV co-infection in 2019 is 11,000. This is approximately 1.9% of the number of TB cases on the same year (WHO 2022). Indeed, the negative impact of synergy between TB and HIV is an alarming public health concern in the Philippines.
The staggering health burden inflicted by TB and HIV co-infection necessitates the use of mathematical modeling to gain insights into its population transmission dynamics, epidemiological patterns, and explore its consequences, among others. Several TB-HIV co-infection models have been developed to obtain understanding on how to prevent new infections, assess the effects of treatment and TB-drug resistance, and explore potential programs that can curb the disease spread in the population (Webster West and Thompson 1997; Schinazi 2003; Naresh and Tripathi 2005; Roeger et al. 2009; Long et al. 2008; Kumar and Jain 2018; Sharomi et al. 2008; Bhunu et al. 2009; Silva and Torres 2015; Mallela et al. 2016; Tasman 2016; Awoke and Semu 2018; Tanvi and Aggarwal 2021; Magombedze et al. 2010; Sergeev et al. 2012). In the current work, a compartmental model of TB and HIV describing its co-infection dynamics is developed, incorporating an unaware PLHIV class. PLHIV who did not know their HIV status contribute significantly to a number of PLHIV who are not seeking medical care and treatment, and who are potential source of new HIV infections (Okosun et al. 2013; Dokubo et al. 2014; Estadilla and de los Reyes 2020). In 2020, about 16% of PLHIV were reported to be incognizant of their HIV status worldwide. In the same year, approximately 32% (twice the global trend) of unaware infectives were reported in the Philippines (UNAIDS 2022). In order to capture the epidemiological dynamics of the dual epidemic in the Philippines, the model is calibrated using available data on TB, HIV, and TB-HIV incidence cases. The parameters estimated include TB and HIV transmission rates, progression rates from exposed to active TB, and from TB-latent with HIV to active infectious TB with HIV in AIDS stage. These parameters substantially affect the increase in the number of co-infected individuals indicated in the sensitivity analysis. In the parameter identification, exponential smoothing is used to give higher weights to the most recent data which are assumed to be collected more reliably. Using bootstrap method, uncertainty analysis is performed to identify the degree of accuracy of parameter estimates. Further, simulation results predict an alarming increase of 180% and 194% in new HIV and TB-HIV infections, respectively, relative to 2019 data, if no efficient control measures will be effectively implemented. These projections accentuate that the Philippines is under health crisis calling for concerted and consolidated efforts to scale-up programs to combat the deadly duo of TB-HIV co-infection.
2 TB-HIV Co-infection Model
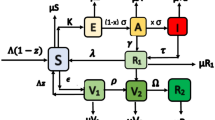
The proposed TB-HIV co-infection model was adapted from the TB dynamical model of Kim et al. (2018), HIV/AIDS model of Estadilla and de los Reyes (2020), and the co-infection model of Bhunu et al. (2009), incorporating classes of individuals unaware of their HIV infection. The model subdivides the human population into susceptible (S), TB-infected, HIV-infected, and TB-HIV co-infected compartments. The TB-infected group is further subdivided into high-risk latent TB individuals \((E_{\textrm{T}})\), symptomatic infectious individuals (\(I_{\textrm{T}}\)), and low-risk latent individuals who recovered from the disease (L). The HIV-infected class include individuals with no clinical symptoms of AIDS who are unaware/aware of their HIV infection \((I_{\textrm{H}_1}/I_{\textrm{H}_2})\), those with AIDS symptoms (A), and those who are under medical treatment for HIV infection \((M_{\textrm{H}})\). The co-infected compartment considers TB-latent individuals with HIV but are unaware/aware of their HIV infection \((E_{\textrm{TH}_1}/E_{\textrm{TH}_2})\), TB infectious individuals with HIV but are unaware/aware of their HIV infection \((I_{\textrm{TH}_1}/I_{\textrm{TH}_2})\), TB infectious individuals with HIV infection having AIDS symptoms \((I_{\textrm{TA}})\), and individuals with TB and HIV under medical treatment for both disease (\(M_{\textrm{TH}}\)). The fourteen mutually exclusive epidemiological classes are depicted in Fig. 1.
Venn diagram of epidemiological classes. The TB-infected group includes \(E_{\textrm{T}}\), \(I_{\textrm{T}}\), and L. Those infected with HIV only are classified into \(I_{\textrm{H}_1},\) \(I_{\textrm{H}_2}\), A, and \(M_{\textrm{H}}\). The co-infected compartments are \(E_{\textrm{TH}_1}\), \(E_{\textrm{TH}_2}\), \(I_{\textrm{TH}_1}\), \(I_{\textrm{TH}_2}\), \(I_{\textrm{TA}}\), and \(M_{\textrm{TH}}\)
The population has a recruitment rate of \(\Lambda \), and all individuals die at a natural rate of \(\mu \). The TB-only compartments are discussed first. Susceptible individuals acquire TB infection after contact with an infectious individual at the rate of \(\lambda _\textrm{T}\). Let \(\beta _\textrm{T}\) be the transmission rate of TB infection. The force of infection associated with TB infection as in Kim et al. (2018) is
A susceptible individual in S who acquires M. tb is moved to the high-risk latent class \(E_{\textrm{T}}\). Individuals in \(E_{\textrm{T}}\) who do not become infectious are transferred to the low-risk latent class L. Since treatment cannot remove tubercle bacilli (i.e., TB-causing bacteria), TB-recovered individuals go from class \(I_{\textrm{T}}\) to class L. In this case, the bacilli remain in the body with a lower risk of progression to active TB (Kim et al. 2020). Note that individuals from \(I_{\textrm{T}}\) may remain infectious after a failed treatment. But for simplicity, we will assume that the treatment is effective so that \(I_{\textrm{T}}\) may only transition to \(E_{\textrm{T}}\) for failed treatment and to L if the treatment is successful. This assumption is based on clinical evidence that effective TB chemotherapy rapidly diminishes the infectiousness of TB patients (Schwartzman and Menzies 2000). In addition, behavioral changes by the TB patients and the people close to them may also contribute to lessening the possibility of TB transmission. TB patients are usually isolated or take necessary precautions when diagnosed with TB, while regular close contact persons also put up safeguards when living with a TB patient undergoing treatment. The complete TB dynamics can be seen in the schematic diagram presented in Fig. 2. This is the TB model in the paper of Kim et al. (2018).
Flow diagram of TB dynamics. Four classes were considered namely: the Susceptible class (S); the high-risk latent TB class (\(E_{\textrm{T}}\)); the TB-infected individuals with symptoms (\(I_{\textrm{T}}\)); and the low-risk latent class (L). The arrows show the flow of individuals from one compartment to another
Before we proceed with the discussion of co-infected compartments, the HIV/AIDS model must first be discussed. HIV is transmitted by an infectious individual to a susceptible person at the rate of \(\lambda _\textrm{H}\). If \(\beta _\textrm{H}\) is the transmission rate of HIV infections, then the force of infection associated with HIV as in Estadilla and de los Reyes (2020) is defined by
Parameters \(\eta _{IH1},\eta _\textrm{H},\) and \(\eta _A\) account for differences in infectiousness of the compartments as infectiousness has a positive relationship with viral loads (Wilson et al. 2008). The modification parameter \(\eta _\textrm{H}<\)1 considers the fact that individuals undergoing HIV treatment are less infectious. Individuals in the AIDS stage are more infectious compared to those who are in non-AIDS stage, which makes \(\eta _A>\)1. Similar to Estadilla and de los Reyes (2020), we will assume that individuals unaware of their HIV infection are more infectious as they are more likely to engage in high-risk behaviors than those who are aware of their infection.
Upon infection, HIV-infected individuals are initially unaware of their disease. A fraction \(\epsilon _1\) of these individuals is screened for HIV. Without treatment, the infection of PLHIV (aware and unaware) in non-AIDS stage will progress to AIDS and these individuals are moved to A at a rate \(\rho _1\). It is assumed here that people who are in the last stage of their HIV infection are already conscious of their disease and are treated at the rate of \(r_5\). Now, the main difference between \(I_{\textrm{H}_1}\) and \(I_{\textrm{H}_2}\) is that the latter group is treated at the rate of \(r_4\) since people in this class are already aware of their infection. There are two treatment failure rates: \(\sigma _1\rho _1\) is the rate of progression to AIDS, where 0\(<\sigma _1<\)1, and \(\omega _1\) is the relapse/withdrawal rate from treatment for PLHIV without AIDS symptoms. PLHIV in AIDS stage die at the rate \(d_A\). The flow diagram of HIV dynamics is presented in Fig. 3.
Flow diagram of HIV/AIDS dynamics. The population was divided to five compartments namely: susceptible (S); HIV-infected individuals with no clinical symptoms of AIDS but unaware of their infection (\(I_{\textrm{H}_1}\)), and those who are aware of their HIV infection (\(I_{\textrm{H}_2}\)); HIV-infected individuals with AIDS symptoms (A); and HIV-infected individuals under medical treatment (\(M_{\textrm{H}}\)). The arrows show the flow of individuals from one compartment to another
The co-infected compartments will now be tackled. Individuals in compartments \(E_{\textrm{TH}_1}\) and \(E_{\textrm{TH}_2}\) who are exposed to TB are moved to \(I_{\textrm{TH}_1}\) and \(I_{\textrm{TH}_2}\), respectively, due to the progression of latent TB to active TB. Their HIV infection may also worsen to AIDS, and in this case, they are transferred to \(I_{\textrm{TA}}\). Screening rates are \(\epsilon _2\) and \(\epsilon _3\) for \(E_{\textrm{TH}_1}\) and \(I_{\textrm{TH}_1}\), respectively. HIV infection of those who are in \(I_{\textrm{TH}_1}\) and \(I_{\textrm{TH}_2}\) may progress to AIDS and they are transferred to \(I_{\textrm{TA}}\) at the rate \(\gamma _2\rho _1\), where \(\gamma _2>1\) because TB intensifies the progression of HIV to AIDS. The co-infected compartments are presented in Fig. 4.
The co-infected compartments. There are six classes, namely: the TB-latent individuals co-infected with HIV but are unaware of their HIV infection (\(E_{\textrm{TH}_1}\)), and those who are aware of their HIV infection (\(E_{\textrm{TH}_2}\)); TB infectious individuals co-infected with HIV but are unaware of their HIV infection (\(I_{\textrm{TH}_1}\)), and those who are aware of their HIV infection (\(I_{\textrm{TH}_2}\)); TB infectious individuals co-infected with HIV infection having AIDS symptoms (\(I_{\textrm{TA}}\)); and individuals co-infected with TB and HIV under medical treatment for both disease (\(M_{\textrm{TH}}\)). The arrows show the flow of individuals from one compartment to another
We will now complete the discussion on TB-HIV/AIDS co-infection model. Let \(\beta _\textrm{T}\) be the transmission rate of TB infection. Incorporating the co-infected compartments, the force of infection associated with TB as in Bhunu et al. (2009), and Awoke and Semu (2018) is
If \(\beta _\textrm{H}\) is the transmission rate of HIV infections, then the force of infection associated with HIV as in Bhunu et al. (2009) and Awoke and Semu (2018) is defined by
where \(H1=I_{\textrm{H}_1}+E_{\textrm{TH}_1}+I_{\textrm{TH}_1}\) and \(H2=I_{\textrm{H}_2}+E_{\textrm{TH}_2}+I_{\textrm{TH}_2}\). A modification parameter for PLHIV in AIDS stage co-infected with active TB is denoted by \(\eta _{TH}>\)1. Starting with the TB infected compartments, high-risk latent individuals (\(E_{\textrm{T}}\)) and individuals with active TB (\(I_{\textrm{T}}\)) may be infected with HIV upon a successful transmission with an infected individual, and thus, they are transferred to \(E_{\textrm{TH}_1}\) and \(I_{\textrm{TH}_1}\), respectively. Individuals in the low-risk latent class (L) who also became infected with HIV are moved to \(I_{\textrm{H}_1}\). On the HIV-related compartments, HIV-infected individuals in classes \(I_{\textrm{H}_1}\) and \(I_{\textrm{H}_2}\) may be exposed to TB at a rate \(\lambda _\textrm{T}\) and are transferred to \(E_{\textrm{TH}_1}\) and \(E_{\textrm{TH}_2}\), respectively. HIV-infected individuals in AIDS stage (A) may be infected with TB and be moved to \(I_{\textrm{TA}}\).
Individuals who are exposed to TB and aware of their HIV infection (\(E_{\textrm{TH}_2}\)) may get both treatments or TB treatment only, because the cost of maintaining ART is higher than TB treatment. The first case means that these individuals are transferred to \(M_{\textrm{TH}}\) and the latter means that they are transferred to \(I_{\textrm{H}_2}\). Although the Philippine’s Department of Health offers free HIV treatment for indigent HIV-infected individuals, only 33% are availing it (Philstar 2017). Those who are in \(E_{\textrm{TH}_1}\) progressed to \(I_{\textrm{H}_1}\) upon availing TB treatment. Individuals with active TB and HIV (\(I_{\textrm{TH}_1},I_{\textrm{TH}_2}\)) may be transferred to \(I_{\textrm{H}_1}\) or \(I_{\textrm{H}_2}\) after a successful TB treatment. On the other hand, a failure in TB treatment means that they are moved to either \(E_{\textrm{TH}_1}\) or \(E_{\textrm{TH}_2}\). Co-infected individuals in AIDS and non-AIDS stage (\(I_{\textrm{TH}_2}, I_{\textrm{TA}}\)) and \(E_{\textrm{TH}_2}\) are transferred to \(M_{\textrm{TH}}\) upon getting both TB and HIV treatments. People under both medications may be transferred to \(E_{\textrm{TH}_2}\) or \(I_{\textrm{TA}}\) which are considered as TB treatment failure for PLHIV co-infected with TB or they may be shifted to \(M_{\textrm{H}}\) upon a successful TB treatment. Figure 5 shows the diagram of the model, and Table 1 lists the values gathered from previous studies on TB, HIV/AIDS, and TB-HIV/AIDS. The recruitment rate \(\Lambda \) and the natural death rate \(\mu \) were estimated using Philippine data.
Model diagram depicting the TB-HIV/AIDS dynamics. There are four groups considered namely: the susceptible individuals, the TB-infected individuals, the HIV-infected individuals, and the individuals co-infected with HIV and TB. The solid lines show the flow of individuals within each group, while the dashed lines show the flow of individuals from one group to another
The TB-HIV/AIDS transmission dynamics is now governed by the following set of ordinary differential equations:
where
3 Materials and Methods
3.1 Epidemiological Data
The TB and TB-HIV incidence data from 2000 to 2019 that were used in this study were downloaded from the WHO TB burden estimates which contains but are not limited to estimates of TB mortality, incidence (which is grouped by age, sex, risk factors, HIV status, rifampicin resistance), case fatality ratio, and treatment coverage (WHO 2022). On the other hand, the records on reported HIV cases were obtained from the Joint United Nations Programme on HIV/AIDS (UNAIDS). This report also provides the number of patients who were initiated on ART, HIV testing, and data on modes of HIV transmission (UNAIDS 2021c). The data on HIV/AIDS from UNAIDS together with the TB incidence and TB-HIV incidence from WHO are used to identify the model parameters in Table 2.
Other data used in this study include life expectancy at birth from 2000 to 2019, which are utilized to estimate the natural death rate. This set of data was obtained from the World Bank’s website (World Bank 2021). The Philippine population in the years 2000 to 2019 which are used to estimate the recruitment rate were gathered from WHO (WHO 2022).
3.2 Estimation of Recruitment and Death Rates
The death rate (\(\mu \)) is computed as the inverse of the mean life expectancy which is calculated using the data in the years 2000–2019 from the World Bank (World Bank 2021). The estimated death rate is 0.014318/year.
The recruitment rate (\(\Lambda \)) was computed by minimizing the sum of the squared errors between the total population and the solution of \(\frac{\hbox {d}N}{\hbox {d}t}\) using the MATLAB function lsqcurvefit. Adding all ODEs yields \(\dfrac{\hbox {d}N}{\hbox {d}t}=\Lambda -\mu N-d_\textrm{T}I.\) Since \(d_\textrm{T}I\) is negligible compared to \(\Lambda \) and \(\mu N,\) then the equation can be approximated as \(\dfrac{\hbox {d}N}{\hbox {d}t}\approx \Lambda -\mu N.\) The estimated value for \(\Lambda \) is 2, 890, 865. The graph of the population data and the estimate is depicted in Fig. 6.
3.3 Stability Analysis
The state variables in the model (5) describe human populations and as such, it is necessary to show that their values remain nonnegative for any time \(t>0\). In addition, the following theorem shows that the region \(\mathscr {M}\) defined by
is time invariant. This means that for the model to be realistic, the state variables should only take values in \(\mathscr {M}\). The details of the proof are provided in “Appendix.”
Theorem 1
If \(S(0),E_{\textrm{T}}(0), \ldots ,M_{\textrm{TH}}(0)\ge 0\), then \(S(t),E_{\textrm{T}}(t), \ldots ,M_{\textrm{TH}}(t)\ge 0\) for all \(t> 0\) and every solution of (5) starting in \({\mathscr {M}}\) remains in \({\mathscr {M}}\) for \(t>0\).
Next, we can find the disease-free equilibrium (DFE) at a point in \(\mathscr {M}\) for which there is no disease in the population, that is,
The DFE of the TB/HIV-AIDS model (5) is
The local stability of DFE (\(Q_0\)) will now be established by initially determining the basic reproduction number \(R_0\). We define \(R_0\) as the expected number of secondary infections produced by an infected individual during his or her entire infectious period (Diekmann et al. 1990). We can then compute \(R_0\) by using the next-generation method proposed by Van den Driessche and Watmough (2002).
Following Van den Driessche and Watmough (2002), the basic reproduction number of TB-HIV/AIDS model is the spectral radius of the next-generation matrix. Thus, the TB-HIV/AIDS-induced reproduction number is
where
and
The reproduction numbers \(R_\textrm{T}\) and \(R_\textrm{H}\) correspond to the reproduction numbers for the TB and HIV/AIDS transmission models, respectively.
We now formally establish the local asymptotic stability of the DFE (\(Q_0)\) in the next theorem. The following relationship between the basic reproduction number, \(R_0\), and the disease-free equilibrium, \(Q_0\), can be established by using Theorem 2 of Van den Driessche and Watmough (2002).
Theorem 2
The disease-free equilibrium point, \(Q_0\), is locally asymptotically stable for \(R_0<1\) and unstable for \(R_0>1\).
A disease is considered an epidemic if \(R_0>1\). The results from Theorem 2 show that a TB or an HIV epidemic will lead to a TB-HIV co-infection epidemic. The value of \(R_0\) for the TB-HIV co-infection is determined in the parameter identification subsection (Sect. 3.5).
3.4 Sensitivity Analysis
Sensitivity analysis is a method used to identify the effect of each parameter in the model outcome. The objective is to identify important inputs of a model and quantify how uncertainty in the input affects model outcomes (Marino et al. 2008). In this research, the model outcomes are the population of HIV-infected with active TB (\(I_{\textrm{TH}_1}+I_{\textrm{TH}_2}+I_{\textrm{TA}}\)), those with active TB only (\(I_{\textrm{T}}\)), and the population of HIV-infected (\(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\)). To identify the parameters with the greatest effects to these outputs, we used a global sensitivity analysis technique called partial rank correlation coefficient (PRCC) which is proven to be the most reliable and efficient sampling-based method. In implementing the PRCC analysis, the Latin Hypercube Sampling (LHS), which is a stratified sampling without replacement technique, is utilized to obtain the input parameter values (Marino et al. 2008).
In the LHS, a uniform distribution generates the values of each parameter which is implemented 10,000 times. The maximum and minimum values of the parameters including the estimated recruitment and death rates are set to \(\pm \, 70\%\) of the nominal values. Same qualitative results were obtained using \(\pm \, 20\%\), \(\pm \,50\%\), and \(\pm \, 90\%\). A dummy parameter is incorporated to ensure the robustness of the analysis. If this dummy has a high sensitivity, the conclusions of the analysis are not reliable. PRCC values ranging from \(-\,1\) to 1 are computed at different time points using the MATLAB function partialcorr. These values were presented using boxplot as shown in Fig. 7. The value of 1 takes a perfect positive linear relationship, while \(-\,1\) means a perfect negative linear relationship and a large absolute PRCC value would mean a large correlation of the parameter with the model outcome, that is, even a small change to a sensitive parameter would have a significant effect to the model output.
PRCC values of the parameters with respect to the population of PLHIV (\(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\)) are illustrated in Fig. 7B. Parameters \(\beta _\textrm{H}\), \(\eta _{IH1}\), and \(\eta _A\) were found to have high positive PRCC values which implies that an increase in these parameters will result to a rise in \(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\). On the other hand, \(r_4\) and \(d_{A}\) have high negative PRCC values suggesting that a positive change in their values will consequently reduce \(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\). Meanwhile, k, \(\beta _\textrm{T}\) and \(\Lambda \) have high positive PRCC values with respect to the population of \(I_{\textrm{T}}\). In contrast, \(\alpha \) and \(r_1\) have high negative PRCC values with respect to the said population. The values are presented in Fig. 7C. Lastly, \(\beta _\textrm{H}\), \(\eta _{IH1}\), \(\beta _\textrm{T}\), k and \(\gamma _1\) were among the parameters with high positive PRCC values, while \(r_1\), \(r_5\), \(r_4\), \(\kappa \) and \(d_{A}\) were found to have high negative PRCC values with respect to the population of PLHIV with active TB (\(I_{\textrm{TH}_1}+I_{\textrm{TH}_2}+I_{\textrm{TA}}\)). The values are shown in Fig. 7A.
PRCC values depicting the sensitivities of the model output A \(I_{\textrm{TH}_1}+I_{\textrm{TH}_2}+I_{\textrm{TA}}\); B \(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\); and C \(I_{\textrm{T}}\) with respect to the model parameters. With respect to the population of PLHIV with active TB (\(I_{\textrm{TH}_1}+I_{\textrm{TH}_2}+I_{\textrm{TA}}\)), \(\beta _\textrm{H}\), \(\eta _{IH1}\), \(\beta _\textrm{T}\), k and \(\gamma _1\) are among the parameters with high positive PRCC values while \(r_1\), \(r_5\), \(r_4\), \(\kappa \) and \(d_{A}\) were found to have negative PRCC values. Parameters \(\beta _\textrm{H}\), \(\eta _{IH1}\), and \(\eta _A\) have high positive PRCC values with respect to the population of PLHIV (\(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A\)), while \(r_4\) and \(d_A\) have negative PRCC values. Parameters k, \(\beta _\textrm{T}\), and \(\Lambda \) and have high positive PRCC values, while \(\alpha \) and \(r_1\) have negative PRCC values with respect to the output \(I_{\textrm{T}}\)
3.5 Parameter Identification
The model output is made as close as possible to the set of observations by estimating some of the parameters indicated in Table 2. Parameter estimation involves minimizing a measure of error between the model output and the data that were gathered. It was determined that the model outputs have high sensitivity values on these parameters which indicate the parameters’ influential effect to the outcomes. Consequently, accurate values must be assigned to these parameters. Furthermore, these parameters are vital to the Philippines’ goal on treating 2.5 million tuberculosis patients (DOH 2020) and to the 2025 AIDS targets of UNAIDS, which includes 95% of all PLHIV know their HIV status, 95% of all people with diagnosed HIV infection initiate treatment, and 95% of all people receiving treatment are virally suppressed (UNAIDS 2021c).
In this study, the simple exponential smoothing was used to give higher weight to the most recent data, that is, weights assigned to observations decrease exponentially as we go to older data. By this, more importance is given to the latest data compared to the earlier ones. Letting \(i=0,1, \ldots ,n-1\), the weight for observation \(t_i\) is
where \(0<\theta <1\) is the rate at which the weights decrease exponentially (Chowell 2017). Here, \(\theta =0.25\) is assigned to TB, HIV and TB-HIV incidence. In this study, the square of differences between the model outcome and the collected data is minimized using MATLAB function fminsearchbnd. Parameters were then bounded to their respective feasible range of values listed in Table 2. The resulting estimated values are also summarized in Table 2, and the plots of model output versus data are illustrated in Fig. 9.
The estimated value for the TB transmission rate \(\beta _\textrm{T}\) is comparable to the estimates of other studies in TB dynamics in the Philippines (Kim et al. 2018; Villasin et al. 2017). The progression rate to active TB (k) is higher compared to the estimate of Kim et al. (2018) which can be attributed to a larger TB incidence estimates used in this study. These higher estimates were brought about by a more accurate TB-testing technology in the country, GeneXpert (Crisostomo 2018). The estimate of TB treatment rate \(r_1\) falls within the interval set in the records of WHO (World Health Organization 2019). The estimated \(\beta _\textrm{H}\) which is the HIV transmission rate is close to the result of another HIV modeling study in the Philippines (Estadilla and de los Reyes 2020). Our estimate of \(\gamma _1\) suggests that latent TB infection intensifies the progression of HIV infection to AIDS. This result is consistent with Sullivan et al. (2015).
In order to quantify the uncertainty of the estimates, the parameter bootstrapping approach is used. In this procedure, multiple samples from the best-fit model are generated (Chowell 2017). Furthermore, accuracy measures are assigned to the sample estimates. Using the method of least-square fitting, the parameters and the best model are identified. By assuming a Poisson and negative binomial error structure, simulated data sets are then generated from the best-fit model. The Poisson distribution is used when the epidemiological data have a mean that is equal to the variance while the negative binomial distribution is more applicable to overdispersed data whose variance is much larger than the mean (Chowell 2017). Since we have limited access to available information on TB-HIV co-infection (only 20 data points), crude estimates on the ratio of variance and the mean for the TB, HIV, and TB-HIV data, are 4900, 3900, and 3500, respectively. In this work, it is assumed that the variance is a thousand times higher than the mean. New parameter estimates will then be obtained from these simulated data sets and these sets of parameters will be utilized to form confidence intervals. Figure 8 shows the resulting distributions from repeatedly sampling the data a thousand times for the Poisson and negative binomial error structures, respectively. Table 2 lists the 95% confidence interval from the distribution for the Poisson error structure and negative binomial error structure.
Bootstrap distributions of the parameter estimates \(\beta _\textrm{H}\), \(\beta _\textrm{T}\), k, \(r_1\), and \(\gamma _1\) from repeatedly sampling the TB, HIV, TB-HIV incidences data a thousand times using A Poisson and B negative binomial error structures. The 95% confidence interval (CI) from the distribution is also indicated
Finally, we estimate the reproduction number (\(R_0\)) of the TB-HIV co-infection. Note that an epidemic occurs when \(R_0\) is greater than 1. Using the next-generation method (Diekmann et al. 1990), the resulting reproduction number is \(R_0=\max \{4.1015, 3.7655\}=4.1015.\) Note that 4.1015 is the reproduction number of the TB model, while 3.7655 is of the HIV model. The higher reproduction number of TB compared to HIV is expected due to its airborne nature of transmission. However, the reproduction number of HIV is relatively close to that of the TB model even though it is more difficult to transmit.
3.6 Projections
Poisson Error Structure Using the estimated parameters in Table 2 and the parameters from previous studies compiled in Table 1, the infection incidences from 2020 to 2025 were forecasted. Figure 9 represents the projections for the TB-HIV co-infection incidence, TB, and HIV incidences if the current intervention efforts in the country remain the same.
Expected number of TB-HIV incidence, TB incidence, and HIV incidence from the model using A Poisson and B negative binomial error structures. The projections show around 166% increase in HIV incidence and a 170% increase in the TB-HIV incidence from 2019 to 2025 for both error structure. However, a wider confidence interval can be observed for negative binomial error structure when data are overdispersed
Percentage increase of TB-HIV incidence, TB incidence, and HIV incidence from 2019 using a Poisson and negative binomial error structure. It is projected that the TB-HIV incidence will increase by around 170% in 2025 and 509% in 2030 compared to the projections in 2019 if the current programs and efforts will not be improved
Comparing the projections in 2019, new TB infections in the country will increase by approximately 6% in 2025. It is also predicted that there will be a 166% increase in new HIV incidence and a 170% increase in the TB-HIV incidence (Fig. 10). Now, if you compare these projections to the actual data in 2019, there will be about 7% additional TB infections, 180% increase in new HIV incidence, and 194% new TB-HIV co-infections in 2025. The latter comparison is higher compared to the projection of 2019 since not all new infections are reported and included in the actual data. Also, note that these increases in new infections are in the span of 6 years only.
Negative Binomial Error Structure Now, assuming a negative binomial error structure that is applicable for overdispersed data, projections were also made. The projection of the best-fit model was not far from the best-fit model with the Poisson error structure. However, the confidence band around the best-fit model is much wider than that of the Poisson error structure because of the assumption of overdispersal (Fig. 10). By this, the forecast can vary widely in this confidence interval depending on how large the variance is compared to the mean of the actual data. In 2025, the lowest number of possible new TB infections is projected to be 561,898 with the highest that can equal to 718,110. For HIV, the lowest projection is 23,021 while the highest number of new HIV infections is 53,353. Finally, for new TB-HIV co-infections, the least possible new incidences is 22,179, but it can go as high as 44,677 new co-infections (Fig. 10).
Our projections for HIV incidence are in line with the projection of the Department of Health (DOH) (Department of Health - Epidemiology Bureau 2020). Based on the DOH’s baseline projection for 2025, there will be 33,400 new HIV cases among individuals aged 15 and above. This is relatively close to this study’s projection of new infections with 39,692 for a Poisson error structure or 38,728 for a negative binomial error structure (Fig. 10). The Philippine Department of Health (DOH) utilizes the AIDS Epidemic Model (AEM) and Spectrum to model HIV estimates and projections (Department of Health - Epidemiology Bureau 2020). The AEM is a process model that considers both behavioral and biological inputs. This semi-empirical, risk-structured model is patterned primarily on the dominant modes of transmission routes for HIV in Asia including (extra)marital sex, sex work, male-male sex, and injecting drug use. Model parameters are adjusted by the user until the output agrees with the observational data. Since each risk class has its corresponding set of parameters, AEM becomes unusable if data from the identified risk classes are lacking. Hence, the complexity of this tool requires careful handling of extensive and reliable epidemiological and behavioral input data (Brown and Peerapatanapokin 2004). For instance, sexual behavior inputs (e.g., number of sexual partners, condom use, etc.,) are based on self-reports that may be biased (King 2022; Stover et al. 2021). On the contrary, the present modeling framework divides the total population into several distinct epidemiological classes (susceptible, (un)aware HIV-infected, etc.). This is considered a transmission dynamic model that aims to project population-based outcomes from individual-level inputs (Cassels et al. 2008). It should be noted that in the current modeling framework, subpopulations (e.g., susceptibles, exposed, infectious, etc.) are assumed to be homogeneous; that is, they are composed of individuals who mix randomly and uniformly with each other. This assumption on homogeneous mixing is analytically tractable but may not reflect the real disease transmission dynamics. This might impose a limitation on the long-term projection of the disease. Here, actual epidemic data is used to calibrate the model, estimating key parameters, reflecting the disease transmission. Both AEM and the current model provide dynamic projections of the HIV epidemic trend in the Philippines. The AEM clearly identifies the source of HIV transmission in the Philippines—men who have sex with men (MSM). An advantage of the transmission model is its capability to assess the impact of specific changes in the model parameters and determine what changes will have the greatest/least influence on the infection through sensitivity analysis. Further, it considers an imperfect data where under- and un-reporting might be present, and uncertainty analysis through bootstrapping method can be performed. The present model can be extended using stratified classes to incorporate different risk groups including marital sex, extramarital sex, sex work, male–male sex, needle sharing, and mother-to-child transmission. Then, comprehensive comparison between AEM and the extended model can be made to improve both approaches for better HIV projections.
4 Conclusion
Tuberculosis remains to be a burden in the Philippines despite various government programs to eliminate the disease. To make it worse, the Philippines has the fastest growing HIV epidemic in the Asia Pacific Region, which is probably aggravated by persons with HIV that are unaware of their infection. As previously emphasized, TB-HIV co-infection is a deadly combination since one infection boosts the other.
A TB-HIV/AIDS co-infection model is developed, incorporating an unaware class of PLHIV where individuals in the class are not treated for HIV infection and are possible cause of new infections. Some of the parameters were estimated based on TB, HIV, and TB-HIV incidences data by initially examining the parameters on which the model outcomes (\(I_{\textrm{T}}\), \(I_{\textrm{H}_1}+I_{\textrm{H}_2}+A,\) and \(I_{\textrm{TH}_1}+I_{\textrm{TH}_2}+I_{\textrm{TA}}\)) are sensitive. Utilizing these estimates together with the available parameter values from previous studies on TB, HIV and TB-HIV co-infection, it was determined that the disease-free equilibrium is locally asymptotically unstable if \(R_0\) is greater than 1, which is the current status of the Philippines based on the analyses presented. So either an epidemic of TB or HIV can lead to an epidemic of their co-infection. By this, it is not enough to address one of this diseases, but both must be tackled head-on with efficient mitigation strategies to avoid the epidemic of TB-HIV co-infection.
Further model iteration should include the relative infectiousness of HIV-positive and HIV-negative TB patients with regard to transmission of M. tb. In particular, Espinal et al. (2000) noted that HIV-positive individuals with TB are less likely to transmit M. tb to their close contacts as compared to TB-infected HIV-negative individuals. Modification parameters associated with \(I_{\textrm{T}}, I_{TH_1}, I_{TH_2},\) and \(I_{TA}\) can be incorporated in the TB force of infection, \(\lambda _\textrm{T}\). These parameters need to be carefully estimated given access to reliable information to account for specific infectiousness of the different groups.
Projections for the years 2020 to 2025 were also presented in this paper (Fig. 9). It is predicted that TB incidence, HIV incidence, and TB-HIV incidence in the country will continuously rise if the current efforts in controlling these diseases will not be improved. Based on these projections, the country’s increase in TB incidence is significantly slower compared to the increase in new HIV infections. Although the percentage increase in the TB incidence is slower, the number of individuals with active TB is still high. Given the already huge number of TB cases, the HIV epidemic in the Philippines will be the driver of the TB and HIV co-infection in the country. This is worsened by the current situation in the country with regard to the programs and services against HIV aggravated by the COVID19 pandemic.
Globally, there has been a reduction of 31% from the new HIV infections and 47% from deaths as compared to 2010 according to the 2021 updates of WHO on HIV estimates. However, in the Philippines, instead of a decrease in infection, there has been a reduction in HIV prevention services, enrollment in HIV care, and HIV testing due to the COVID19 pandemic. According to an article published in the UNAIDS website (UNAIDS 2021a), HIV prevention services decreased by 74% for men having sex with men in 2019 in Metro Manila, HIV testing decreased by 61% in 2020, while registration in HIV care reduced by 28% in the same year. Furthermore, out of 120, 000 estimated PLHIV in 2020, there are 78,291 individuals diagnosed with HIV. This is barely 66% of the estimated PLHIV. Out of at least 78,000 individuals diagnosed with HIV, there are only 47,977 people on ART or almost 62% only. Finally, there are only 7706 PLHIV who have viral suppression. That is only 16% of individuals on ART (UNAIDS 2021b). This calls for intensifying the strategies in mitigating HIV in the country.
Individuals that are unaware of their infection with HIV, must be identified and treated since this group has the ability to spread the disease at a higher rate. Furthermore, those under medications must be closely monitored to ensure that they are following their treatment carefully. It is also necessary that the public is well-informed of the disease to prevent or minimize its spread. Encouraging those who are vulnerable to the disease to enroll in pre-exposure prophylaxis (PrEP) must also be intensified.
The analyses that were performed here greatly depend on TB and HIV/AIDS data obtained from WHO and UNAIDS. However, the authors observed that data that were gathered may be under-reported or inconsistent since data from both local and international sources were both examined. A better data gathering strategy will be vital to improve the accuracy of the estimated parameters in the model presented in this study. As a consequence, a better projection can be provided. The current modeling study have estimated only 5 parameters that significantly influence the model output (i.e., number of infectious TB and/or HIV individuals) and impact the mitigation programs in reducing TB and/or HIV. Model projections are dependent on the uncertainty of these 5 identified parameters. Better estimates, projections and uncertainty analysis can be obtained provided access to sufficient and reliable information. For future studies, the authors are planning to develop a control model to determine if an optimal control strategy can be determined to fight the spread of TB-HIV co-infection.
Availability of data and materials
The data that support the findings of this study are available from the WHO, UNAIDS, and World Bank. Data from the simulations and simulation codes are, however, available from the authors upon reasonable request.
References
Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A et al (2010) Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med 362(8):697–706. https://doi.org/10.1056/NEJMoa0905848
Awoke TD, Semu MK (2018) Optimal control strategy for TB-HIV/AIDS co-infection model in the presence of behaviour modification. Processes 6(5):48. https://doi.org/10.3390/pr6050048
Bhunu C, Garira W, Mukandavire Z (2009) Modeling HIV/AIDS and tuberculosis coinfection. Bull Math Biol 71(7):1745–1780. https://doi.org/10.1007/s11538-009-9423-9
Brown T, Peerapatanapokin W (2004) The Asian epidemic model: a process model for exploring HIV policy and programme alternatives in Asia. Sex Transm Infect 80(suppl 1):i19–i24. https://doi.org/10.1136/sti.2004.010165
Bruchfeld J, Correia-Neves M, Källenius G (2015) Tuberculosis and HIV coinfection. Cold Spring Harbor Perspect Med 5(7):a017871–a017871. https://doi.org/10.1101/cshperspect.a017871
Cassels S, Clark SJ, Morris M (2008) Mathematical models for HIV transmission dynamics: tools for social and behavioral science research. J Acquir Immune Defic Syndr 47(Suppl 1):S34-9. https://doi.org/10.1097/QAI.0b013e3181605da3
CDC. HIV/AIDS. https://www.cdc.gov/hiv/basics/whatishiv.html
Chaisson RE, Churchyard GJ (2010) Recurrent tuberculosis: relapse, reinfection, and HIV. J Infect Dis 201:653–655. https://doi.org/10.1086/650531
Chowell G (2017) Fitting dynamic models to epidemic outbreaks with quantified uncertainty: a primer for parameter uncertainty, identifiability, and forecasts. Infect Dis Model 2(3):379–398. https://doi.org/10.1016/j.idm.2017.08.001
Crisostomo S (2018) DOH: Philippines has highest tuberculosis cases in ASEAN. The Philippine Star. https://www.philstar.com/headlines/2018/09/26/1854836/doh-philippines-has-highest-tuberculosis-cases-asean
Department of Health (2019) Philippines TB joint program review. https://ntp.doh.gov.ph/download/joint-program-review-2019-report/
Department of Health (2020) Updated Philippine strategic TB elimination plan phase 1: 2020–2023. https://doh.gov.ph/node/24443
Department of Health - Epidemiology Bureau (2020) A briefer on the Philippine HIV estimates. https://doh.gov.ph/sites/default/files/publications/A%20Briefer%20on%20the%20PH%20Estimates%202020_08232021.pdf
Diedrich CR, Flynn JL (2011) HIV-1/mycobacterium tuberculosis coinfection immunology: how does HIV-1 exacerbate tuberculosis? Infect. Immun. 79(4):1407–1417. https://doi.org/10.1128/IAI.01126-10
Diekmann O, Heesterbeek JAP, Metz JA (1990) On the definition and the computation of the basic reproduction ratio \(R_0\) in models for infectious diseases in heterogeneous populations. J Math Biol 28(4):365–382. https://doi.org/10.1007/BF00178324
DOH (2020) National tuberculosis program manual of procedures 6th edition. https://doh.gov.ph/sites/default/files/publications/NTP_MOP_6th_Edition.pdf
Dokubo EK, Shiraishi RW, Young PW, Neal JJ, Aberle-Grasse J, Honwana N et al (2014) Awareness of HIV status, prevention knowledge and condom use among people living with HIV in Mozambique. PLoS ONE 9(9):1–6. https://doi.org/10.1371/journal.pone.0106760
Espinal MA, Peréz EN, Baéz J, Hénriquez L, Fernández K, Lopez M et al (2000) Infectiousness of Mycobacterium tuberculosis in HIV-1-infected patients with tuberculosis: a prospective study. Lancet 355(9200):275–280
Estadilla CDS, de los Reyes AA (2020) Optimal strategies for mitigating the HIV/AIDS epidemic in the Philippines. Math Methods Appl Sci 43(18):10690–10710. https://doi.org/10.1002/mma.6979
Gangcuangco LMA (2019) HIV crisis in the Philippines: urgent actions needed. Lancet Public Health 4(2):E84. https://doi.org/10.1016/S2468-2667(18)30265-2
Getahun H, Gunneberg C, Granich R, Nunn P (2010) HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50(Supplement-3):S201–S207. https://doi.org/10.1086/651492
HIV/AIDS Data hub. TB-HIV Factsheet 2018 Asia and the Pacific. https://www.aidsdatahub.org/sites/default/files/highlight-reference/document/DataHub_TB-HIV_Fact_Sheet_2018.pdf
King BM (2022) The influence of social desirability on sexual behavior surveys: a review. Arch Sex Behav 51(3):1495–1501. https://doi.org/10.1007/s10508-021-02197-0
Kim S, Aurelio A, Jung E (2018) Mathematical model and intervention strategies for mitigating tuberculosis in the Philippines. J Theor Biol 443:100–112. https://doi.org/10.1016/j.jtbi.2018.01.026
Kim S, de los Reyes VAA, Jung E (2020) Country-specific intervention strategies for top three TB burden countries using mathematical model. PLoS ONE 15(4):964e0230
Kumar S, Jain S (2018) Assessing the effects of treatment in HIV-TB co-infection model. Eur Phys J Plus 133(8):294. https://doi.org/10.1140/epjp/i2018-12117-8
Kwan C, Ernst JD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24(2):351–376. https://doi.org/10.1128/CMR.00042-10
Long EF, Vaidya NK, Brandeau ML (2008) Controlling co-epidemics: analysis of HIV and tuberculosis infection dynamics. Oper Res 56(6):1366–1381. https://doi.org/10.1287/opre.1080.0571
Magombedze G, Garira W, Mwenje E (2010) Modeling the TB/HIV-1 co-infection and the effects of its treatment. Math Popul Stud 17(1):12–64
Mallela A, Lenhart S, Vaidya NK (2016) HIV-TB co-infection treatment: modeling and optimal control theory perspectives. J Comput Appl Math 307:143–161. https://doi.org/10.1016/j.cam.2016.02.051
Marino S, Hogue IB, Ray CJ, Kirschner DE (2008) A methodology for performing global uncertainty and sensitivity analysis in systems biology. J Theor Biol 254(1):178–196. https://doi.org/10.1016/j.jtbi.2008.04.011
Mesfin YM, Hailemariam D, Biadglign S, Kibret KT (2014) Association between HIV/AIDS and multi-drug resistance tuberculosis: a systematic review and meta-analysis. PLoS ONE 9(1):e82235. https://doi.org/10.1371/journal.pone.0082235
Modjarrad K, Vermund SH (2010) Effect of treating co-infections on HIV-1 viral load: a systematic review. Lancet Infect Dis 10(7):455–463. https://doi.org/10.1016/S1473-3099(10)70093-1
Naresh R, Tripathi A (2005) Modelling and analysis of HIV-TB co-infection in a variable size population. Math Model Anal 10(3):275–286
Okosun KO, Makinde OD, Takaidza I (2013) Impact of optimal control on the treatment of HIV/AIDS and screening of unaware infectives. Appl Math Model 37(6):3802–3820. https://doi.org/10.1016/j.apm.2012.08.004
Pawlowski A, Jansson M, Sköld M, Rottenberg ME, Källenius G (2012) Tuberculosis and HIV co-infection. PLoS Pathog 8(2):1–7. https://doi.org/10.1371/journal.ppat.1002464
Philstar. DOH: only 33% of HIV positive Pinoys seek free treatment due to stigma. https://www.philstar.com/headlines/2017/12/01/1764282/doh-only-33-hiv-positive-pinoys-seek-free-treatment-due-stigma
Roeger LIW, Feng Z, Castillo-Chávez C (2009) Modeling TB and HIV co-infections. Math Biosci Eng 6(4):815–837. https://doi.org/10.3934/mbe.2009.6.815
Schinazi RB (2003) Can HIV invade a population which is already sick? Bull Braz Math Soc 34(3):479–488. https://doi.org/10.1007/s00574-003-0025-0
Schwartzman K, Menzies D (2000) How long are TB patients infectious? CMAJ 163(2):157–158
Sergeev R, Colijn C, Murray M, Cohen T (2012) Modeling the dynamic relationship between HIV and the risk of drug-resistant tuberculosis. Sci Transl Med 4(135):135ra67
Sharomi O, Podder CN, Gumel AB, Song B (2008) Mathematical analysis of the transmission dynamics of HIV/TB coinfection in the presence of treatment. Math Biosci Eng 5(1):145–174. https://doi.org/10.3934/mbe.2008.5.145
Silva CJ, Torres DF (2015) A TB-HIV/AIDS coinfection model and optimal control treatment. arXiv:1501.03322
Stover J, Glaubius R, Teng Y, Kelly S, Brown T, Hallett TB et al (2021) Modeling the epidemiological impact of the UNAIDS 2025 targets to end AIDS as a public health threat by 2030. PLoS Med 18(10):1–21. https://doi.org/10.1371/journal.pmed.1003831
Sullivan ZA, Wong EB, Ndung’u T, Kasprowicz VO, Bishai WR (2015) Latent and active tuberculosis infection increase immune activation in individuals co-infected with HIV. EBioMedicine 2(4):334–340. https://doi.org/10.1016/j.ebiom.2015.03.005
Tanvi, Aggarwal R (2021) Estimating the impact of antiretroviral therapy on HIV-TB co-infection: optimal strategy prediction. Int J Biomath 14(01):2150004. https://doi.org/10.1142/S1793524521500042
Tasman H et al (2016) An optimal treatment control of TB-HIV coinfection. Int J Math Math Sci. https://doi.org/10.1155/2016/8261208
UNAIDS (2021a) Community-led HIV services stepped up in the Philippines during the COVID-19 pandemic. https://www.unaids.org/en/keywords/philippines
UNAIDS (2021b) Philippines key facts on HIV. https://www.aidsdatahub.org/country-profiles/philippines
UNAIDS (2021c) 2025 AIDS Targets. https://aidstargets2025.unaids.org/#section-targets
UNAIDS (2022) Global data on HIV epidemiology and response. https://aidsinfo.unaids.org/
Van den Driessche P, Watmough J (2002) Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math Biosci 180(1–2):29–48. https://doi.org/10.1016/S0025-5564(02)00108-6
Villasin KJB, Lao AR, Rodriguez EM (2017) A dynamical analysis of tuberculosis in the Philippines. Philipp Sci Lett 10(1):29–37
Webster West R, Thompson JR (1997) Modeling the impact of HIV on the spread of tuberculosis in the United States. Math Biosci 143(1):35–60. https://doi.org/10.1016/S0025-5564(97)00001-1
WHO (2019) It’s time to end TB in the Philippines. https://www.who.int/philippines/news/commentaries/detail/it-s-time-to-end-tb-in-the-philippines#:~:text=Tuberculosis%20is%20an%20airborne%20disease.%20About%201%20million,the%20number%20one%20killer%20among%20all%20infectious%20diseases
WHO (2022) Global tuberculosis programme. https://www.who.int/teams/global-tuberculosis-programme/data
WHO Europe (2006) Management of tuberculosis and HIV coinfection: clinical protocol for the WHO European region
Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM (2008) Relation between HIV viral load and infectiousness: a model-based analysis. Lancet 372(9635):314–320. https://doi.org/10.1016/S0140-6736(08)61115-0
World Bank (2021) Life expectancy at birth, total (years). Available from: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=PH
World Health Organization (2018) Tuberculosis. World Health Organization. http://www.wpro.who.int/philippines/areas/communicable_diseases/tb/story_continuation_tb_area_page/en/
World Health Organization (2019) Tuberculosis. https://www.who.int/tb/country/data/download/en/
World Health Organization (2021) Global Tuberculosis Report 2021. Licence: CC BY-NC-SA 3.0 IGO
World Health Organization (2022a) Tuberculosis. https://www.who.int/news-room/fact-sheets/detail/tuberculosis
World Health Organization (2022b) HIV/AIDS. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
Acknowledgements
The authors want to express their gratitude to the University of the Philippines Enhanced Creative Work and Research Grant (UP ECWRG) for funding this research. ADLRV acknowledges the support of the Institute of Mathematics, University of the Philippines Diliman and the Institute for Basic Science (IBS-R029-C3). JMT and MCT are grateful for the support of the Mathematics Division, Institute of Mathematics Sciences and Physics, University of the Philippines Los Baños.
Funding
This work was funded by the UP System Enhanced Creative Work and Research Grant (ECWRG-2020-2-33R).
Author information
Authors and Affiliations
Contributions
All authors conceived and designed the study, MT executed the study, and all authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare that we have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A Proof
Theorem 1. If \(S(0),E_{\textrm{T}}(0), \ldots ,M_{\textrm{TH}}(0)\ge 0\) then \(S(t),E_{\textrm{T}}(t), \ldots ,M_{\textrm{TH}}(t)\ge 0\) for all \(t> 0\) and every solution of (5) starting in \({\mathscr {M}}\) remains in \({\mathscr {M}}\) for \(t>0\).
Proof
Let \(S(0),E_{\textrm{T}}(0), \ldots ,M_{\textrm{TH}}(0)\ge 0\). Let
For the first part, it is enough to show that
at the boundaries of \({\mathbb {R}}_+^{14}\). We will prove this by contradiction.
Suppose there exists first time \(t_1,t_2, \ldots ,t_{14}>0\) such that any of the following occurs,
but \(\Lambda >0\), a contradiction.
but \(\lambda _\textrm{T}S(t_2)+(1-p_1)r_1I_{\textrm{T}}(t_2)\ge 0\) since \(0<p_1<1\). Another contradiction.
but \(kE_{\textrm{T}}(t_3)\ge 0\) which results another contradiction. Similar arguments can be applied to solve for \(L'(t_4), I_{\textrm{H}_1}'(t_5), \ldots ,M_{\textrm{TH}}'(t_{14})\) that will lead to contradictions.
These imply that there is no such \(t_1,t_2,t_3, \ldots ,t_{14}\). Hence,
for all \(t>0\).
Now for the second part, the rate of change of the total population obtained by adding all equations in (5) is
Since
then
Hence, N(t) is less than or equal to the solution of
Let \(N(0)=N_0\). Then,
Lastly, if \(N_0\le \dfrac{\Lambda }{u}\), then \(N(t)\le \dfrac{\Lambda }{u}\) since \(\hbox {e}^{-\mu t}>0\).
Therefore, every solution of (5) starting in \({\mathscr {M}}\) remains in \({\mathscr {M}}\) for \(t>0\). \(\square \)
Appendix B Analysis of the Submodels
Stability analysis of TB-only and HIV-only models is discussed here.
1.1 B.1 TB Submodel
The TB submodel (when no HIV/AIDS disease), i.e.,
is given by:
where
with force of infection
This is the TB model presented in Kim et al. (2018).
1.1.1 B.1.1 Positivity and Boundedness of Solutions
Based on biological considerations, the system (B15) will be studied in the following region:
The system of equations (B15) describes human populations and for it to be epidemiologically meaningful, it is necessary to show that all state variables \(S(t),E_{\textrm{T}}(t),I_{\textrm{T}}(t),\) and \(L_\textrm{T}(t)\) are nonnegative for all time \(t>0\). This also means the solution of (B15) with nonnegative initial values must remain nonnegative for all time t. The next theorem will show that \({\mathscr {M}}_1\) is positively invariant with respect to the system (B15).
Theorem 3
If \(S(0),E_{\textrm{T}}(0),I_{\textrm{T}}(0),L(0)\ge 0\) then \(S(t),E_{\textrm{T}}(t),I_{\textrm{T}}(t),L(t)\ge 0\) for all \(t> 0\). Moreover, every solution of (B15) starting in \({\mathscr {M}}_1\) remains in \({\mathscr {M}}_1\) for \(t>0\).
Proof
Let \(S(0),E_{\textrm{T}}(0),I_{\textrm{T}}(0),L(0)\ge 0\). Let \(X=(S,E_{\textrm{T}},I_{\textrm{T}},L)\). For the first part, it is enough to show that
at the boundaries of \({\mathbb {R}}_+^{4}\). We will prove this by contradiction.
Suppose there exist first time \(t_1,t_2,t_3,t_{4}>0\) such that
Note that every parameter used in the TB submodel is positive. From (B15) and (B16),
but \(\Lambda >0\), a contradiction.
but \(\lambda _{T1}S(t_2)+(1-p_1)r_1I_{\textrm{T}}(t_2)\ge 0\) since \(0<p_1<1\). Another contradiction.
but \(kE_{\textrm{T}}(t_3)\ge 0\) which results to another contradiction.
but \(\alpha E_{\textrm{T}}(t_4)+p_1r_1I_{\textrm{T}}(t_4)\ge 0\).
These imply that there is no such \(t_1,t_2,t_3\) and \(t_4\). Hence, (for all \(t>0\)),
Now for the second part, the rate of change of the total population obtained by adding all equations in (B15) is
Since
then
Hence, N(t) is less than or equal to the solution of
Let \(N(0)=N_0\). Then,
Lastly, if \(N_0\le \dfrac{\Lambda }{u}\) then \(N(t)\le \dfrac{\Lambda }{u}\) since \(\hbox {e}^{-\mu t}>0\).
Therefore, every solution of (B15) starting in \({\mathscr {M}}_1\) remains in \({\mathscr {M}}_1\) for \(t>0\).\(\square \)
1.1.2 B.1.2 Local Stability of Disease Free Equilibrium
The disease-free equilibrium (DFE) is a point in which there is no disease in the population, i.e., \(E_{\textrm{T}}=I_{\textrm{T}}=L=0\). The TB submodel (B15) has a DFE which is obtained by equating the right-hand side to 0. Thus, the disease-free equilibrium is
The local stability of \(Q_\textrm{T}\) will now be established by initially determining the basic reproduction number. We can compute the basic reproduction number, \(R_\textrm{T}\) by using next-generation method proposed in Van den Driessche and Watmough (2002). Following Van den Driessche and Watmough (2002), the basic reproduction number of TB model is the spectral radius of the next-generation matrix \(FV^{-1}\) where,
Thus, the TB-induced reproduction number is
We now formally establish the local asymptotic stability of the DFE (\(Q_\textrm{T})\). By Theorem 2 of Van den Driessche and Watmough (2002), we establish the following relationship between the basic reproduction number, \(R_\textrm{T}\), and the disease-free equilibrium, \(Q_\textrm{T}\).
Theorem 4
The disease-free equilibrium point, \(Q_\textrm{T}\), is locally asymptotically stable for \(R_\textrm{T}<1\) and unstable for \(R_\textrm{T}>1\).
1.2 B.2 HIV/AIDS Submodel
The HIV submodel (when no TB disease), i.e.,
is given by:
where
with force of infection
This is the HIV/AIDS model presented in Estadilla and de los Reyes (2020).
1.2.1 B.2.1 Positivity and Boundedness of Solutions
Based on biological considerations, the system (B20) will be studied in the following region:
The system of equations (B20) describes human populations and for it to be epidemiologically meaningful, it is necessary to show that all state variables S(t), \(I_{\textrm{H}_1}(t),\) \(I_{\textrm{H}_2}(t),\) A(t), and \(M_{\textrm{H}}(t)\) are nonnegative for all time \(t>0\). This also means that the solution of (B20) with nonnegative initial values must remain nonnegative for all time t. The next theorem will show that \({\mathscr {M}}_2\) is positively invariant with respect to the system (B20).
Theorem 5
If \(S(0),I_{\textrm{H}_1}(0),I_{\textrm{H}_2}(0),A(0),M_{\textrm{H}}(0)\ge 0\) then S(t), \(I_{\textrm{H}_1}(t),\) \(I_{\textrm{H}_2}(t),\) A(t), \(M_{\textrm{H}}(t)\) \(\ge 0\) for all \(t> 0\) and every solution of (B20) starting in \({\mathscr {M}}_2\) remains in \({\mathscr {M}}_2\) for \(t>0\).
Proof
Let \(S(0),I_{\textrm{H}_1}(0),I_{\textrm{H}_2}(0),A(0),M_{\textrm{H}}(0)\ge 0\). Let \(X=(S,I_{\textrm{H}_1},I_{\textrm{H}_2},A,M_{\textrm{H}}).\)
For the first part, it is enough to show that
at the boundaries of \({\mathbb {R}}_+^{5}\). We will prove this by contradiction.
Suppose there exists first time \(t_1>0\) such that for \(0\le t\le t_1\),
or there exists first time \(t_2>0\) such that for \(0\le t\le t_2\),
or there exists first time \(t_3>0\) such that for \(0\le t\le t_3\),
or there exists first time \(t_4>0\) such that for \(0\le t\le t_4\),
or there exists first time \(t_5>0\) such that for \(0\le t\le t_5\),
but \(\Lambda >0\) a contradiction.
but \(\lambda _{H1}S(t_2)\ge 0\), another contradiction.
but \(\epsilon _1I_{\textrm{H}_1}(t_3)+\omega _1M_{\textrm{H}}(t_3)\ge 0\) which results to another contradiction.
but \(\rho _1(I_{\textrm{H}_1}(t_4)+I_{\textrm{H}_2}(t_4))+\sigma _1\rho _1M_{\textrm{H}}(t_4)\ge 0\).
Finally, from (B20) and (B25),
but \(r_4I_{\textrm{H}_2}(t_5)+r_5A(t_5)>0\) which results to contradiction.
These imply that there is no such \(t_1,t_2,t_3,t_4\) and \(t_5\). Hence, (for all \(t>0\)),
Now for the second part, the rate of change of the total population obtained by adding all equations in (B20) is
Since
then
Hence, N(t) is less than or equal to the solution of
Let \(N(0)=N_0\). Then,
Lastly, if \(N_0\le \dfrac{\Lambda }{u}\) then \(N(t)\le \dfrac{\Lambda }{u}\) since \(\hbox {e}^{-\mu t}>0\).
Therefore, every solution of (B20) starting in \({\mathscr {M}}_2\) remains in \({\mathscr {M}}_2\) for \(t>0\).\(\square \)
1.2.2 B.2.2 Local Stability of Disease Free Equilibrium
The disease-free equilibrium (DFE) is a point in which there is no disease in the population, i.e., \(I_{\textrm{H}_1}=I_{\textrm{H}_2}=A=M_{\textrm{H}}=0\). The HIV/AIDS submodel (B20) has a DFE which is obtained by equating the right-hand side to 0. Thus, the disease-free equilibrium is
The local stability of \(Q_\textrm{H}\) will now be established by initially determining the basic reproduction number. We can compute the basic reproduction number, \(R_\textrm{H}\) by using next-generation method proposed in Van den Driessche and Watmough (2002). Following Van den Driessche and Watmough (2002), the basic reproduction number of HIV/AIDS model is the spectral radius of the next-generation matrix \(FV^{-1},\) where
and
Thus, the HIV/AIDS-induced reproduction number is
and
We now formally establish the local asymptotic stability of the DFE (\(Q_\textrm{H})\). By Theorem 2 of Van den Driessche and Watmough (2002), we establish the following relationship between the basic reproduction number, \(R_\textrm{H}\), and the disease-free equilibrium, \(Q_\textrm{H}\).
Theorem 6
The disease-free equilibrium point, \(Q_\textrm{H}\), is locally asymptotically stable for \(R_\textrm{H}<1\) and unstable for \(R_\textrm{H}>1\).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, M., Tubay, J. & de losReyes, A. Quantitative Assessment of a Dual Epidemic Caused by Tuberculosis and HIV in the Philippines. Bull Math Biol 85, 56 (2023). https://doi.org/10.1007/s11538-023-01156-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01156-1