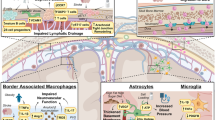
Abstract
The renin angiotensin system (RAS), which is classically known for blood pressure regulation, has functions beyond this. There are two axes of RAS that work to counterbalance each other and are active throughout the body, including the CNS. The pathological axis, consisting of angiotensin II (A1-8), angiotensin converting enzyme (ACE) and the angiotensin II type 1 receptor (AT1R), is upregulated in many CNS diseases, including multiple sclerosis (MS). MS is an autoimmune and neurodegenerative disease of the CNS characterized by inflammation, demyelination and axonal degeneration. Published research has described increased expression of AT1R and ACE in tissues from MS patients and in animal models of MS such as experimental autoimmune encephalomyelitis (EAE). In contrast to the pathological axis, little is known about the protective axes of RAS in MS and EAE. In other neurological conditions the protective axis, which includes A1–7, ACE2, angiotensin II type 2 receptor and Mas receptor, has been shown to have anti-inflammatory, regenerative and neuroprotective effects. Here we show, for the first time, changes in the protective arm of RAS in both EAE and MS CNS tissue. We observed a significant increase in expression of the protective arm during stages of disease stabilization in EAE, and in MS tissue showing evidence of remyelination. These data provide evidence that the protective arm of RAS, through both ligand and receptor expression, is associated with reductions in the pathological processes that occur in the earlier stages of MS and EAE, possibly slowing the neurodegenerative process and enhancing neural repair.

Graphical Abstract






Similar content being viewed by others
References
Acuna MJ et al (2014) Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-beta signalling. Hum Mol Genet 23:1237–1249. https://doi.org/10.1093/hmg/ddt514
Ahmad S, Varagic J, Groban L, Dell’Italia LJ, Nagata S, Kon ND, Ferrario CM (2014) Angiotensin-(1-12): a chymase-mediated cellular angiotensin II substrate. Curr Hypertens Rep 16:429. https://doi.org/10.1007/s11906-014-0429-9
Allen AM, Moeller I, Jenkins TA, Zhuo J, Aldred GP, Chai SY, Mendelsohn FA (1998) Angiotensin receptors in the nervous system. Brain Res Bull 47:17–28
Bader M (2013) ACE2, angiotensin-(1-7), and Mas: the other side of the coin. Pflugers Arch 465:79–85
Bennion DM, Haltigan E, Regenhardt RW, Steckelings UM, Sumners C (2015) Neuroprotective mechanisms of the ACE2-angiotensin-(1-7)-Mas axis in stroke. Curr Hypertens Rep 17:3. https://doi.org/10.1007/s11906-014-0512-2
Bjartmar C, Wujek JR, Trapp BD (2003) Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 206:165–171
Bodiga VL, Bodiga S (2013) Renin angiotensin system in cognitive function and dementia. Asian J Neurosci 2013:18. https://doi.org/10.1155/2013/102602
Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE (2011) Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond) 121:297–303. https://doi.org/10.1042/CS20110036
Charcot JM (1868) Histologie de la sclerose en plaques. La Lancette Française Gazette des Hopitaux Civil et Militaires 41:554
Ciobica A, Bild W, Hritcu L, Haulica I (2009) Brain renin-angiotensin system in cognitive function: pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurol Belg 109:171–180
Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164:1079–1106. https://doi.org/10.1111/j.1476-5381.2011.01302.x
de Kloet AD, Liu M, Rodriguez V, Krause EG, Sumners C (2015) Role of neurons and glia in the CNS actions of the renin-angiotensin system in cardiovascular control. Am J Physiol Regul Integr Comp Physiol 309:R444–R458. https://doi.org/10.1152/ajpregu.00078.2015
Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI (1997) Counterregulatory actions of angiotensin-(1-7). Hypertension 30:535–541
Frischer JM et al (2009) The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain 132:1175–1189. https://doi.org/10.1093/brain/awp070
Gironacci MM, Longo Carbajosa NA, Goldstein J, Cerrato BD (2013) Neuromodulatory role of angiotensin-(1-7) in the central nervous system. Clin Sci (Lond) 125:57–65. https://doi.org/10.1042/CS20120652
Goodin DS (2014) The epidemiology of multiple sclerosis: insights to disease pathogenesis. Handb Clin Neurol 122:231–266. https://doi.org/10.1016/B978-0-444-52001-2.00010-8
Guo X, Namekata K, Kimura A, Harada C, Harada T (2017) The renin-angiotensin system regulates neurodegeneration in a mouse model of optic neuritis. Am J Pathol 187:2876–2885. https://doi.org/10.1016/j.ajpath.2017.08.012
Hammer A et al (2016) Role of the receptor Mas in macrophage-mediated inflammation in vivo. Proc Natl Acad Sci U S A 113:14109–14114. https://doi.org/10.1073/pnas.1612668113
Jiang T, Gao L, Lu J, Zhang YD (2013a) ACE2-Ang-(1-7)-Mas axis in brain: a potential target for prevention and treatment of ischemic stroke. Curr Neuropharmacol 11:209–217. https://doi.org/10.2174/1570159X11311020007
Jiang T, Gao L, Shi J, Lu J, Wang Y, Zhang Y (2013b) Angiotensin-(1-7) modulates renin-angiotensin system associated with reducing oxidative stress and attenuating neuronal apoptosis in the brain of hypertensive rats. Pharmacol Res 67:84–93. https://doi.org/10.1016/j.phrs.2012.10.014
Kawajiri M et al (2009) Angiotensin-converting enzyme (ACE) and ACE2 levels in the cerebrospinal fluid of patients with multiple sclerosis. Mult Scler 15:262–265. https://doi.org/10.1177/1352458508097923
Kostenis E et al (2005) G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation 111:1806–1813. https://doi.org/10.1161/01.CIR.0000160867.23556.7D
Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H (2017) An updated histological classification system for multiple sclerosis lesions. Acta Neuropathol 133:13–24. https://doi.org/10.1007/s00401-016-1653-y
Lanz TV et al (2010) Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120:2782–2794. https://doi.org/10.1172/JCI41709
Lu J et al (2013) The expression of angiotensin-converting enzyme 2-angiotensin-(1-7)-Mas receptor axis are upregulated after acute cerebral ischemic stroke in rats. Neuropeptides 47:289–295. https://doi.org/10.1016/j.npep.2013.09.002
Lund BT et al (2019) Reduced disease severity following therapeutic treatment with angiotensin 1-7 in a mouse model of multiple sclerosis. Neurobiol Dis 127:87–100. https://doi.org/10.1016/j.nbd.2019.02.018
Magalhaes GS et al (2015) Angiotensin-(1-7) attenuates airway remodelling and hyperresponsiveness in a model of chronic allergic lung inflammation. Br J Pharmacol 172:2330–2342. https://doi.org/10.1111/bph.13057
Mario EG, Santos SH, Ferreira AV, Bader M, Santos RA, Botion LM (2012) Angiotensin-(1-7) Mas-receptor deficiency decreases peroxisome proliferator-activated receptor gamma expression in adipocytes. Peptides 33:174–177. https://doi.org/10.1016/j.peptides.2011.11.014
McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen R, Oldfield BJ, Mendelsohn FA, Chai SY (2003) The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35:901–918
Mogi M, Horiuchi M (2013) Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr Gerontol Int 13:13–18. https://doi.org/10.1111/j.1447-0594.2012.00900.x
Namsolleck P et al (2013) AT2-receptor stimulation enhances axonal plasticity after spinal cord injury by upregulating BDNF expression. Neurobiol Dis 51:177–191. https://doi.org/10.1016/j.nbd.2012.11.008
Ocaranza MP et al (2014) Angiotensin-(1-9) reverses experimental hypertension and cardiovascular damage by inhibition of the angiotensin converting enzyme/Ang II axis. J Hypertens 32:771–783. https://doi.org/10.1097/HJH.0000000000000094
Oliveira-Lima OC et al (2015) Mas receptor deficiency exacerbates lipopolysaccharide-induced cerebral and systemic inflammation in mice. Immunobiology 220:1311–1321. https://doi.org/10.1016/j.imbio.2015.07.013
Passos-Silva DG, Verano-Braga T, Santos RA (2013) Angiotensin-(1-7): beyond the cardio-renal actions. Clin Sci (Lond) 124:443–456. https://doi.org/10.1042/CS20120461
Platten M et al (2009) Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A 106:14948–14953. https://doi.org/10.1073/pnas.0903958106
Procaccini C, De Rosa V, Pucino V, Formisano L, Matarese G (2015) Animal models of multiple sclerosis. Eur J Pharmacol 759:182–191. https://doi.org/10.1016/j.ejphar.2015.03.042
Rabelo LA, Xu P, Todiras M, Sampaio WO, Buttgereit J, Bader M, Santos RA, Alenina N (2008) Ablation of angiotensin (1-7) receptor Mas in C57Bl/6 mice causes endothelial dysfunction. J Am Soc Hypertens 2:418–424. https://doi.org/10.1016/j.jash.2008.05.003
Rangachari M, Kuchroo VK (2013) Using EAE to better understand principles of immune function and autoimmune pathology. J Autoimmun 45:31–39. https://doi.org/10.1016/j.jaut.2013.06.008
Regenhardt RW, Desland F, Mecca AP, Pioquinto DJ, Afzal A, Mocco J, Sumners C (2013) Anti-inflammatory effects of angiotensin-(1-7) in ischemic stroke. Neuropharmacology 71:154–163. https://doi.org/10.1016/j.neuropharm.2013.03.025
Rice GI, Thomas DA, Grant PJ, Turner AJ, Hooper NM (2004) Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J 383:45–51. https://doi.org/10.1042/BJ20040634
Santos RA et al (2003) Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A 100:8258–8263. https://doi.org/10.1073/pnas.1432869100
Stegbauer J et al (2009) Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci U S A 106:14942–14947. https://doi.org/10.1073/pnas.0903602106
Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM (2013) Protective arms of the renin-angiotensin-system in neurological disease. Clin Exp Pharmacol Physiol 40:580–588. https://doi.org/10.1111/1440-1681.12137
Trapp BD, Nave KA (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci 31:247–269. https://doi.org/10.1146/annurev.neuro.30.051606.094313
Valero-Esquitino V et al (2015) Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin Sci (Lond) 128:95–109. https://doi.org/10.1042/CS20130601
Wright JW, Harding JW (2013) The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflugers Arch 465:133–151. https://doi.org/10.1007/s00424-012-1102-2
Xu P et al (2008) Endothelial dysfunction and elevated blood pressure in MAS gene-deleted mice. Hypertension 51:574–580. https://doi.org/10.1161/HYPERTENSIONAHA.107.102764
Zhao Y, Qin Y, Liu T, Hao D (2015) Chronic nerve injury-induced Mas receptor expression in dorsal root ganglion neurons alleviates neuropathic pain. Exp Ther Med 10:2384–2388. https://doi.org/10.3892/etm.2015.2801
Acknowledgements
This work was supported by the Department of Defense (Grant No. MS130053 – BTL). MS brain specimens were obtained from the Human Brain and Spinal Fluid Resource Center, which is sponsored by NINDS/NIMH, National Multiple Sclerosis Society, VA Greater Los Angeles Healthcare System, 11301 Wilshire Blvd. Los Angeles, CA 90073, and Veterans Health Services and Research Administration, Department of Veterans Affairs.
Funding
This work was supported by the Department of Defense (Grant No. MS130053 - BTL).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This animal study was performed in accordance with ethical and humane standards set forth by the National Institutes of Health, and with the approval of University of Southern California’s Institutional Animal Care and Use Committee.
Conflict of Interest
All of the authors of this manuscript declare no conflict of interest. Dr. Lund has however received investigator-initiated funding from Teva Pharmaceutical Industries, Novartis Pharmaceuticals Corporation and Sanofi-Genzyme for projects unrelated to this work. Dr. Lund has also received honoraria from Teva for projects unrelated to this work. Dr. Kelland has received investigator-initiated funding from Teva Pharmaceutical Industries and Novartis Pharmaceuticals Corporation for projects unrelated to this work. Drs. Rodgers, Stone, Liu, Levy, Kashani and Louie have no industry related conflicts regarding research in multiple sclerosis. Drs. Lund, Kelland, Louie and Rodgers are inventors of U.S. Patent No. 9,623,084.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in these studies involving animals were in accordance with ethical and humane standards set forth by the National Institutes of Health, and with the approval of University of Southern California’s Institutional Animal Care and Use Committee. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stone, R.E., Liu, S., Levy, A.M. et al. Activation of the Protective Arm of the Renin Angiotensin System in Demyelinating Disease. J Neuroimmune Pharmacol 15, 249–263 (2020). https://doi.org/10.1007/s11481-019-09894-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09894-7