Abstract
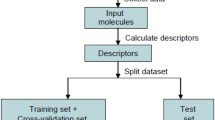
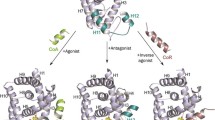
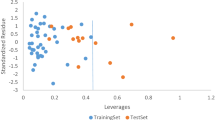
Particular non-monotonic dose-response curves of many endocrine disrupting chemicals (EDCs) suggest the existence of diverse toxicity mechanisms at different dose levels. As a result, the biological activities of EDCs cannot be simply exhibited by unique EC 50/LD 50 values, and the quantitative structure-activity relationship (QSAR) analysis for non-monotonic dose-response relationship becomes an unknown field in the environmental science. In this paper, nine phenols with inverted U-shaped dose-response curves in lymphocyte proliferation test of Carassius auratus were selected. The binding interactions between the phenols and several typical EDCs-related receptors were then explored in a molecular simulation study. The estrogen receptor (ER), androgen receptor (AR), thyroid hormone receptor (TR), bacterial O2 sensing FixL protein (FixL), aryl hydrocarbon receptor (AhR), and the peroxisome proliferator-activated receptor (PPAR) were the target receptors in the study. Linear regression QSAR models for the low and high exposure levels of the compounds were developed separately. The results indicated that the lymphocyte proliferation in the low-dose range might involve ER-mediated process, while the proliferation inhibition in the high dose range was dominated by the acute toxicity of phenols due to receptor occupancy and cell damage.
Similar content being viewed by others
References
vom Saal F S, Timms B G, Montano M M, et al. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. PNAS, 1997, 94: 2056–2061
Wetherill Y B, Petre C E, Monk K R, et al. The xenoestrogen bisphenol A induces inappropriate androgen receptor activation and mitogenesis in prostatic adenocarcinoma cells. Mol Cancer Ther, 2002, 1: 515–524
vom Saal F S, Nagel S C, Palanza P, et al. Estrogenic pesticides: binding relative to estradiol in MCF-7 cells and effects of exposure during fetal life on subsequent territorial behaviour in male mice. Toxicol Lett, 1995, 77(1): 343–350
Calabrese E J, Baldwin L A. Hormesis as a biological hypothesis. Environ Health Perspect, 1998, 106(suppl1): 357–362
Calabrese E J. Estrogen and related compounds: Biphasic dose responses. Crit Rev Toxicol, 2001, 31(4–5): 503–515
Calabrese E J, Baldwin L A. The hormesis dose-response model is more common than the threshold model in toxicology. Toxicol Sci, 2003, 71(2): 246–250
Calabrese E J, Cook R R. Hormesis: How it could affect the risk assessment process. Human Exp Toxicol, 2005, 24(5): 265–270
Cook R R, Calabrese E J. The importance of hormesis to public health. Environ Health Perspect, 2006, 114(11): 1631–1635
Wang D H, Peng A, Wang Z J. Advances in study of hormesis (in Chinese). J Safety Environ, 2004, 4(1): 18–21
Liu S S, Liu F, Liu H L. Toxicities of 20 kinds of water-soluble organic solvents to Vibrio-qinghaiensis sp. Q67 (in Chinese). Chin Environ Sci, 2007, 27(3): 371–376
Stebbing A R D. A theory for growth hormesis. Mut Res Fund Mol Mechan Mutagen, 1998, 403(1): 249–258
Stebbing A R D. Hormesis: Interpreting the beta-curve using control theory. J Appl Toxicol, 2000, 20(2): 93–101
Zala S M, Penn D J. Abnormal behaviours induced by chemical pollution: A review of the evidence and new challenges. Anim Behav, 2004, 68(4): 649–664
Asikainen A, Ruuskanen J, Tuppurainen K. Consensus kNN QSAR: A versatile method for predicting the estrogenic activity of organic compoundsin silico. A comparative study with five estrogen receptors and a large, diverse set of ligands. Environ Sci Technol, 2004, 38(24): 6724–6729
Wang Y W, Liu H X, Zhao C Y, et al. Quantitative structure-activity relationship models for prediction of the toxicity of polybrominated diphenyl ether congeners. Environ Sci Technol, 2005, 39(13): 4961–4966
Rubin B S, Murray M K, Damassa D A, et al. Perinatal exposure to low doses of bisphenol A affects body weight, patterns of estrous cyclicity, and plasma LH levels. Environ Health Perspect, 2001, 109(7): 675–680
Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med, 2000, 224: 61–68
Coser K R, Chesnes J, Hur J Y, et al. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. PNAS, 2003, 100(24): 13994–13999
Michalik L, Auwerx J, Berger JP, et al. International union of pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev, 2006, 58(4): 726–741
Feige J N, Gelman L, Michalik L, et al. From molecular action to physiological outputs: peroxisome proliferator-activated receptors are nuclear receptors at the crossroads of key cellular functions. Prog Lipid Res, 2006, 45 (2): 120–159
Li Y, Hu S Q, Yin D Q, et al. Primary screening and evaluation of endocrine disrupting activities of eleven substituted phenols (in Chinese). Environ Chem, 2003, 22(4): 385–389
Brian J V, Harris C A, Scholze M, et al. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect, 2005, 113(6): 721–728
Costache A D, Pullela P K, Kasha P, et al. Homology-modeled ligand-binding domains of zebrafish estrogen receptors α, β1, and β2: from in silico to in vivo studies of estrogen interactions in danio rerio as a model system. Mol Endocrinol, 2005, 19(12): 2979–2990
Ikeuchi T, Todo T, Kobayashi T, et al. cDNA cloning of a novel androgen receptor subtype. J Biol Chem, 1999, 274(36): 25205–25209
Maglich J M, Caravella J A, Lambert M H, et al. The first completed genome sequence from a teleost fish (Fugu rubripes) adds significant diversity to the nuclear receptor superfamily. Nucl Acids Research, 2003, 31(14): 4051–4058
Zoete V, Grosdidier A, Michielin O. Peroxisome proliferator-activated receptor structures: ligand specificity, molecular switch and interactions with regulators. Biochim Biophys Acta, 2007, 1771(8): 915–925
Folkertsma S, van Noort P, Van Durme J, et al. A family-based approach reveals the function of residues in the nuclear receptor ligand-binding domain. J Mol Biol, 2004, 341(2): 321–335
Procopio M, Lahm A, Tramontano A, et al. A model for recognition of polychlorinated dibenzo-p-dioxins by the aryl hydrocarbon receptor. FEBS J, 2002, 269(1): 13–18
van Lipzig M M H, ter Laak A M, Jongejan A, et al. Prediction of ligand binding affinity and orientation of xenoestrogens to the estrogen receptor by molecular dynamics simulations and the linear interaction energy method. J Med Chem, 2004, 47(4): 1018–1030
Lund I A. A monte carlo method for testing the statistical significance of a regression equation. J Appl Meteor, 1970, 9(3): 330–332
Summers J K, Wison H T, Kou J. A method for quantifying the prediction uncertainties associated with water quality models. Ecological Modelling, 1993, 65(3–4): 161–176
Pandini A, Denison M S, Song Y, et al. Structural and functional characterization of the aryl hydrocarbon receptor ligand binding domain by homology modeling and mutational analysis. Biochemistry, 2007, 46 (3): 696–708
Denison M S, Pandini A, Nagy S R, et al. Ligand binding and activation of the Ah receptor. Chem Biol Inter, 2002, 141(1): 3–24
Jones D T. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol, 1999, 292(2): 195–202
Rost B. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol, 1996, 266: 525–539
Zhang J F, Liu H, Sun Y Y, et al. Responses of the antioxidant defenses of the Goldfish Carassius auratus, exposed to 2,4-dichlorophenol. Environ Toxicol Pharm, 2005, 19(1): 185–190
Welshons W V, Thayer K A, Judy B M, et al. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect, 2003, 111(8): 994–1006
MacLusky N J, Hajszan T, Leranth C. The environmental estrogen bisphenol-A inhibits estrogen-induced hippocampal synaptogenesis. Environ Health Perspect, 2005, 113(6): 675–679
vom Saal F S, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect, 2005, 113(8): 926–933
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 20777035 and 20377022) and National High Technology Research and Development Program of China (Grant Nos. 2007AA06Z416 and 2006AA06Z424)
About this article
Cite this article
Gao, C., Zhang, A., Lin, Y. et al. Quantitative structure-activity relationships of selected phenols with non-monotonic dose-response curves. Chin. Sci. Bull. 54, 1786–1796 (2009). https://doi.org/10.1007/s11434-009-0174-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-009-0174-7