Abstract
Purpose
Pharmaceutical residues in the environment can pose significant risks to ecosystems and human beings due to adverse pharma-specific effects. Existing life cycle assessment (LCA) studies do not usually consider the use and end-of-life (EoL) phase of pharmaceuticals and thus exclude relevant potentially toxic emissions of an active pharmaceutical ingredient (API). Therefore, a simplified inventory model for the use and EoL phase of pharmaceuticals is provided by estimating API flows and emissions to the environment.
Methods
Both the qualitative description of the use and EoL phase of pharmaceuticals and the quantification of the flows within each life cycle phase are based on literature and expert knowledge. Existing approaches to determine the API emissions are adjusted to make them applicable in LCA. In addition, different uses and EoL scenarios (e.g. depending on the patients’ disposal behaviour) are specified, and assumptions are highlighted. Finally, the model is exemplarily applied to the oral intake of ibuprofen to test its applicability.
Results and discussion
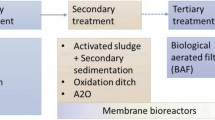
Eleven potential flows and emissions of an API are identified and quantified for different application forms (pulmonary, oral, cutaneous). The model is applied to ibuprofen where potential API emissions result from administered and unused products. Referred to the administered amount of ibuprofen (reference flow), the product is mainly metabolized (73.1%). The unmetabolized (parental) compound enters the sewage treatment plant where it is degraded (13.94%), or emitted to surface water (8.35%), air (0%) and sewage sludge (0.36%). The remainder cannot be clearly assigned to one of the flows (4.25%). The results of this example are hardly comparable to existing measured data because they are related to the functional unit. The effect of assumptions, limitations due to data availability and the geographic scope reveal the need for further research.
Conclusions
To facilitate the consideration of the use and EoL phase of pharmaceuticals in future pharma-LCAs, a simplified inventory model specified for German conditions, is provided which allows to calculate inventory results with easily and publically accessible data. However, remaining challenges such as the lack of data to model the behaviour of metabolites in the sewage treatment plant, missing approaches to include specific pharmaceuticals (e.g. hormones, anticancer drugs), the consideration of other sewage treatment technologies such as ozonization, the integration of API emissions from sewage sludge (e.g. due to the use as fertilizer) or the scope expansion with regard to the geographic validity of the model shall be further examined.


Similar content being viewed by others
Change history
28 April 2020
The original version of this article unfortunately contained a mistake which was missed during typesetting.
References
Aguirre-Martínez GV, Owuor MA, Garrido-Pérez C, Salamanca MJ, Del Valls TA, Martín-Díaz ML (2015) Are standard tests sensitive enough to evaluate effects of human pharmaceuticals in aquatic biota? Facing changes in research approaches when performing risk assessment of drugs. Chemosphere 120:75–85. https://doi.org/10.1016/j.chemosphere.2014.05.087
Aktories K, Forth W (2013) Allgemeine und spezielle Pharmakologie und Toxikologie. Für Studenten der Medizin, Veterinärmedizin, Pharmazie, Chemie und Biologie sowie für Ärzte, Tierärzte und Apotheker ; mit 305 Tabellen ; [Plus im Web, mediscript]. 11. überarb. Aufl. München: Elsevier Urban & Fischer. Available online at http://www.sciencedirect.com/science/book/9783437425233
Al Aukidy M, Verlicchi P, Jelic A, Petrovic M, Barcelò D (2012) Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci Total Environ 438:15–25. https://doi.org/10.1016/j.scitotenv.2012.08.061
Alder AC, Schaffner C, Majewsky M, Klasmeier J, Fenner K (2010) Fate of beta-blocker human pharmaceuticals in surface water: comparison of measured and simulated concentrations in the Glatt Valley Watershed, Switzerland. Water Res 44(3):936–948. https://doi.org/10.1016/j.watres.2009.10.002
Arnold KE, Brown AR, Ankley GT, Sumpter JP (2014) Medicating the environment: assessing risks of pharmaceuticals to wildlife and ecosystems. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 369 (1656). DOI: https://doi.org/10.1098/rstb.2013.0569
Bartsch J (2010) Umfrage zum Umgang mit alten Arzneimitteln. Hanau
Belboom S, Renzoni R, Verjans B, Léonard A, Germain A (2011) A life cycle assessment of injectable drug primary packaging. Comparing the traditional process in glass vials with the closed vial technology (polymer vials). Int J Life Cycle Assess 16(2):159–167. https://doi.org/10.1007/s11367-011-0248-z
Besse J-P, Kausch-Barreto C, Garric J (2008) Exposure assessment of pharmaceuticals and their metabolites in the aquatic environment. Application to the French situation and preliminary prioritization. Hum Ecol Risk Assess Int J 14(4):665–695. https://doi.org/10.1080/10807030802235078
Besse J-P, Latour J-F, Garric J (2012) Anticancer drugs in surface waters: what can we say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ Int 39(1):73–86. https://doi.org/10.1016/j.envint.2011.10.002
BMG (2018) Arzneimittel richtig aufbewahren und entsorgen. Edited by Federal Ministry of Health. Berlin/Bonn. Available online at https://www.bundesgesundheitsministerium.de/arzneimittelentsorgung-und-aufbewahrung.html, checked on 1/16/2019
BMU (2019) General information waste water: sewage treatment plant. Edited by Federal Ministry for the Environment, Nature Conversation and Nuclear Safety. Berlin. Available online at https://www.bmu.de/en/topics/water-waste-soil/water-management/wastewater/sewage-treatment-plant/, checked on 1/10/2019
BPB (2012) Vom Hersteller zu den Verbraucherinnen und Verbrauchern: Zulassung, Herstellung und Vertrieb von Arzneimitteln. Edited by Bundeszentrale für politische Bildung. Bonn. Available online at http://www.bpb.de/politik/innenpolitik/gesundheitspolitik/72757/vom-hersteller-zum-verbraucher?p=all, checked on 12/7/2018
Braatz A (2019) Disposal of pharmaceuticals in a mechanical-biological treatment plant. E-Mail (04.04.2019) to Maya Yavor. Nauen, 2019
Brandt KK, Amézquita A, Backhaus T, Boxall A, Coors A, Heberer T, Lawrence JR, Lazorchak J, Schönfeld J, Snape JR, Zhu YG, Topp E (2015) Ecotoxicological assessment of antibiotics: a call for improved consideration of microorganisms. Environ Int 85:189–205. https://doi.org/10.1016/j.envint.2015.09.013
Bund (2014) Wohin mit abgelaufenen Medikamenten? Edited by Federal Government of Germany. Berlin. Available online at https://www.bundesregierung.de/breg-de/aktuelles/wohin-mit-abgelaufenen-medikamenten%2D%2D348852, checked on 1/16/2019
Bund (2019) Siebzehnte Verordnung zur Durchführung des Bundes-Immissionsschutzgesetzes über die Verbrennung und die Mitverbrennung von Abfällen. 17. BImSchV, revised 5/2/2013. Source: Umwelt-online. Available online at https://www.umwelt-online.de/regelwerk/luft/bimschg/vo/17bv_ges.htm, checked on 1/16/2019
BVL (2018) Product category rules for pharmaceutical products and processes. Distribution of pharmaceuticals to patients at home or in a healthcare facility. Oral communication (06.11.2018) to Marc-William Siegert. Berlin, 2018
Caldwell (2016) Sources of pharmaceutical residues in the environment and their control. In: Hester R, Harrison R (eds) Pharmaceuticals in the Environment, vol 41. The Royal Society of Chemistry (Issues in Environmental Science and Technology), London, pp 92–119
Carballa M, Omil F, Lema JM (2008) Comparison of predicted and measured concentrations of selected pharmaceuticals, fragrances and hormones in Spanish sewage. Chemosphere 72(8):1118–1123. https://doi.org/10.1016/j.chemosphere.2008.04.034
Celiz MD, Tso J, Aga DS (2009) Pharmaceutical metabolites in the environment: analytical challenges and ecological risks. Environ Toxicol Chem 28(12):2473–2484. https://doi.org/10.1897/09-173.1
Celle-Jeanton H, Schemberg D, Mohammed N, Huneau F, Bertrand G, Lavastre V, Le Coustumer P (2014) Evaluation of pharmaceuticals in surface water: reliability of PECs compared to MECs. Environ Int 73:10–21. https://doi.org/10.1016/j.envint.2014.06.015
Chèvre N, Coutu S, Margot J, Wynn H, Bader H-P, Scheidegger R, Rossi L (2013) Substance flow analysis as a tool for mitigating the impact of pharmaceuticals on the aquatic system. Water Res 47(9):2995–3005. https://doi.org/10.1016/j.watres.2013.03.004
Christensen FM (1998) Pharmaceuticals in the environment--a human risk? Regul Toxicol Pharmacol : RTP 28(3):212–221. https://doi.org/10.1006/rtph.1998.1253
Cook SM, VanDuinen BJ, Love NG, Skerlos SJ (2012) Life cycle comparison of environmental emissions from three disposal options for unused pharmaceuticals. Environ Sci Technol 46(10):5535–5541. https://doi.org/10.1021/es203987b
Daughton CG, Ruhoy IS (2009) Environmental footprint of pharmaceuticals: the significance of factors beyond direct excretion to sewers. Environ Toxicol Chem 28(12):2495–2521. https://doi.org/10.1897/08-382.1
Davies NM (1998) Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet 34(2):101–154. https://doi.org/10.2165/00003088-199834020-00002
DECHEMA (2019) Arzneimittel - richtig entsorgt. Edited by DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V. Frankfurt a.M. Available online at http://www.arzneimittelentsorgung.de/#section2, checked on 1/10/2019
DIMDI (2019) Anatomisch-therapeutisch-chemische-Klassifikation mit Tagesdosen. Amtliche Fassung des ATC-Index mit DDD-Angaben für Deutschland im Jahre 2019. With assistance of Uwe Fricke, Judith Günther, Katja Niepraschk-von Dollen, Anette Zawinell. Edited by Deutsches Institut für Medizinische Dokumentation und information (DIMDI). Köln. Available online at https://www.dimdi.de/dynamic/.downloads/arzneimittel/atcddd/atc-ddd-amtlich-2019.pdf, checked on 1/8/2019
DUH (2013) Altmedikamente verantwortungsbewusst entsorgen! Hintergrundpapier zum umweltschonenden Umgang mit abgelaufenen Medikamenten. Edited by Deutsche Umwelthilfe. Hannover. Available online at www.duh.de/uploads/media/Altmedikamente_Hintergrundpapier_12S_01.pdf, checked on 1/9/2019
Ebele AJ, Abou-Elwafa AM, Harrad S (2017) Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerging Contaminants 3(1):1–16. https://doi.org/10.1016/j.emcon.2016.12.004
ECHA (2019) QSAR models. Edited by European chemicals agency. Available online at https://echa.europa.eu/support/registration/how-to-avoid-unnecessary-testing-on-animals/qsar-models, checked on 7/3/2019
Efferth T (2006) Molekulare Pharmakologie und Toxikologie. Biologische Grundlagen von Arzneimitteln und Giften. Berlin, Heidelberg: Springer-Verlag Berlin Heidelberg (Springer-Lehrbuch). Available online at http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10157781
Emara Y, Lehmann A, Siegert M-W, Finkbeiner M (2018) Modeling pharmaceutical emissions and their toxicity-related effects in life cycle assessment (LCA): a review. Integr Environ Assess Manag. https://doi.org/10.1002/ieam.4100
EMA (2006) Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use. First version. Edited by European Medicines Agency. London. Available online at https://www.ema.europa.eu/en/environmental-risk-assessment-medicinal-products-human-use, checked on 12/4/2018
EMA (2018) Guideline on the environmental risk assessment of medicinal products for human use. Draft. Edited by European Medicines Agency. London. Available online at https://www.ema.europa.eu/documents/scientific-guideline/draft-guideline-environmental-risk-assessment-medicinal-products-human-use-revision-1_en.pdf, checked on 1/8/2019
EU (2000) Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the incineration of waste. Edited by European Parliament and the Council. Brussels. Available online at https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0076, checked on 1/30/2019
European Commission (2003) Technical Guidane Document on Risk Assessment. Part I & II. In support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances and Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market. Edited by European Commission. Brussels. Available online at https://ec.europa.eu/jrc/en/publication/eur-scientific-and-technical-research-reports/technical-guidance-document-risk-assessment-part-1-part-2, checked on 1/15/2019
European Commission (2018) Product Environmental Footprint Category Rules Guidance. Version 6.3. Edited by European Commission. Brussels. Available online at ec.europa.eu/environment/eussd/smgp/pdf/PEFCR_guidance_v6.3.pdf, checked on 1/11/2019
Franco A, Struijs J, Gouin T, Price OR (2013a) Evolution of the sewage treatment plant model SimpleTreat: applicability domain and data requirements. Integrated Environ Assess Manage 9(4):560–568. https://doi.org/10.1002/ieam.1414
Franco A, Struijs J, Gouin T, Price OR (2013b) Evolution of the sewage treatment plant model SimpleTreat: use of realistic biodegradability tests in probabilistic model simulations. Integr Environ Assess Manag 9(4):569–579. https://doi.org/10.1002/ieam.1413
Gilroy EAM, Klinck JS, Campbell SD, McInnis R, Gillis PL, de Solla SR (2014) Toxicity and bioconcentration of the pharmaceuticals moxifloxacin, rosuvastatin, and drospirenone to the unionid mussel Lampsilis siliquoidea. Sci Total Environ 487:537–544. https://doi.org/10.1016/j.scitotenv.2014.03.051
Gómez-Canela C, Miller TH, Bury NR, Tauler R, Barron LP (2016) Targeted metabolomics of Gammarus pulex following controlled exposures to selected pharmaceuticals in water. Sci Total Environ 562:777–788. https://doi.org/10.1016/j.scitotenv.2016.03.181
Götz K, Keil F (2007) Arzneimittel in der Umwelt. Medikamentenentsorgung in privaten Haushalten: Ein Faktor bei der Gewässerbelastung mit Arzneimittelwirkstoffen? In UWSF - Z Umweltchem Ökotox 19(3):180–188. https://doi.org/10.1065/uwsf2007.07.205
Han EJ, Kim HS, Lee DS (2014) An emission model tracking the life cycle pathways of human pharmaceuticals in Korea. Environ Health Prev Med 19(1):46–55. https://doi.org/10.1007/s12199-013-0352-8
Han J, Lee DS (2017) Significance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci Total Environ 592:600–607. https://doi.org/10.1016/j.scitotenv.2017.03.044
Heberer T (2002) Occurence, fate, and removal of pharmaceutical residues in the aquatic environment: a review of recent research data. Toxicol Lett 131:5–17
Hirsch R (2019) Pharmacokinetic behavior of APIs for pulmonary applications. Oral communication (27.02.2019) to Marc-William Siegert. Cologne/Berlin, 2019
Hörsing M, Ledin A, Grabic R, Fick J, Tysklind M, La Cour JJ, Andersen HR (2011) Determination of sorption of seventy-five pharmaceuticals in sewage sludge. Water Res 45(15):4470–4482. https://doi.org/10.1016/j.watres.2011.05.033
ISO (2006a) Environmental management – life cycle assessment – principles and framework (ISO 14040:2006). International Organization for Standardization, Geneva
ISO (2006b) Environmental management – Life cycle assessment – Requirements and guidelines (ISO 14044:2006). International Organization for Standardization, Geneva.
ISOE (2008): Pharmaceuticals for Human Use: Options of Action for Reducing the Contamination of Water Bodies - A practical guide. Edited by Florian Keil. Institute for Social-Ecological Research (ISOE) GmbH. Research Project start. Frankfurt a.M. Available online at https://www.isoe.de/uploads/media/start-brochure-2008-en.pdf, checked on 12/6/2018
Jelic A, Gros M, Ginebreda A, Cespedes-Sánchez R, Ventura F, Petrovic M, Barcelo D (2011) Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res 45(3):1165–1176. https://doi.org/10.1016/j.watres.2010.11.010
Johnson AC, Williams RJ (2004) A model to estimate influent and effluent concentrations of estradiol, estrone, and ethinylestradiol at sewage treatment works. Environ Sci Technol 38(13):3649–3658. https://doi.org/10.1021/es035342u
Jones O, Voulvoulis N, Lester J (2002) Aquatic environmental assessment of the top 25 English prescription pharmaceuticals. Water Res 36(20):5013–5022. https://doi.org/10.1016/S0043-1354(02)00227-0
Khan SJ, Ongerth JE (2004) Modelling of pharmaceutical residues in Australian sewage by quantities of use and fugacity calculations. Chemosphere 54(3):355–367. https://doi.org/10.1016/j.chemosphere.2003.07.001
Kinrys G, Gold AK, Worthington JJ, Nierenberg AA (2018) Medication disposal practices: increasing patient and clinician education on safe methods. J Int Med Res 46(3):927–939. https://doi.org/10.1177/0300060517738681
Klöpffer W, Grahl B (2009) Ökobilanz (LCA). Ein Leitfaden für Ausbildung und Beruf Weinheim: WILEY-VCH Available online at http://lib.myilibrary.com?id=213988
Kookana RS, Williams M, Boxall ABA, Larsson D GJ, Gaw S, Choi K et al (2014) Potential ecological footprints of active pharmaceutical ingredients: an examination of risk factors in low-, middle- and high-income countries. In Philosophical transactions of the Royal Society of London. Series B, Biological Sciences 369 (1656). DOI: https://doi.org/10.1098/rstb.2013.0586
Kostich MS, Lazorchak JM (2008) Risks to aquatic organisms posed by human pharmaceutical use. Sci Total Environ 389(2–3):329–339. https://doi.org/10.1016/j.scitotenv.2007.09.008
Kümmerer K, Schuster A, Längin A, Happel O, Thoma A, Schneider K et al (2011) Identifizierung und Bewertung ausgewählter Arzneimittel und ihrer Metaboliten (Ab- und Umbauprodukte) im Wasserkreislauf. Edited by German Environment Agency. Dessau-Roßlau. Available online at https://www.umweltbundesamt.de/en/publikationen/identifizierung-bewertung-ausgewaehlter, checked on 1/11/2019
Landry KA, Boyer TH (2016) Life cycle assessment and costing of urine source separation: focus on nonsteroidal anti-inflammatory drug removal. Water Res 105:487–495. https://doi.org/10.1016/j.watres.2016.09.024
Langford K, Thomas KV (2011) Input of selected human pharmaceutical metabolites into the Norwegian aquatic environment. J Environ Monit JEM 13(2):416–421. https://doi.org/10.1039/C0EM00342E
Langner A, Borchert H-H, Mehnert W, Pfeifer S (2011) Biopharmazie. Pharmakokinetik - Bioverfügbarkeit - Biotransformation. 4., völlig neu bearb. und erw. Aufl. Stuttgart: Wiss. Verl.-Ges
Lautz LS, Struijs J, Nolte TM, Breure AM, van der Grinten E, van de Meent D, van Zelm R (2017) Evaluation of SimpleTreat 4.0: simulations of pharmaceutical removal in wastewater treatment plant facilities. Chemosphere 168:870–876. https://doi.org/10.1016/j.chemosphere.2016.10.123
Li WC (2014) Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut (Barking, Essex: 1987) 187:193–201. https://doi.org/10.1016/j.envpol.2014.01.015
Mackay D (1979) Finding fugacity feasible. Environ Sci Technol 13(10):1218–1223 Available online at https://pubs.acs.org/doi/pdf/10.1021/es60158a003 , checked on 1/15/2019
Martin LR, Williams SL, Haskard KB, Dimatteo MR (2005) The challenge of patient adherence. Ther Clin Risk Manag 1(3):189–199
Maplecroft (2019) Waste generation and recycling indices 2019 - overview and findings. Edited by Verisk Maplecroft. Available online at https://www.circularonline.co.uk/wp-content/uploads/2019/07/Verisk_Maplecroft_Waste_Generation_Index_Overview_2019.pdf, checked on 10/15/2019
Masoner JR, Kolpin DW, Furlong ET, Cozzarelli IM, Gray JL (2016) Landfill leachate as a mirror of today's disposable society: pharmaceuticals and other contaminants of emerging concern in final leachate from landfills in the conterminous United States. Environ Toxicol Chem 35(4):906–918. https://doi.org/10.1002/etc.3219
Medsafe (2017): Ibuprofen New Zealand Data Sheet. Edited by Medsafe New Zealand Medicines and Medical Devices Safety Authority. Wellington. Available online at https://www.medsafe.govt.nz/profs/datasheet/i/IbuprofenArrowcaretab.pdf, checked on 1/14/2018
Mompelat S, Le Bot B, Thomas O (2009) Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ Int 35(5):803–814. https://doi.org/10.1016/j.envint.2008.10.008
Monteiro SC, Boxall AB (eds) (2010) Occurence and fate of human pharmaceuticals in the environment. In: Whitacre D (ed) Reviews of environmental contamination and toxicology. Volume 202. ebrary, Inc. New York: Springer (reviews of environmental contamination and toxicology, 202). Available online at http://site.ebrary.com/lib/alltitles/docDetail.action?docID=10351752
NHS (2012) Greenhouse gas accounting sector guidance for pharmaceutical products and medical devices. Full Guidance. Edited by National Health Service. London. Available online at https://www.sduhealth.org.uk/areas-of-focus/carbon-hotspots/pharmaceuticals.aspx, checked on 1/9/2019
O'Brien JW, Banks APW, Novic AJ, Mueller JF, Jiang G, Ort C et al (2017) Impact of in-sewer degradation of pharmaceutical and personal care products (PPCPs) population markers on a population model. Environ Science Technol 51(7):3816–3823. https://doi.org/10.1021/acs.est.6b02755
Ong TTX, Blanch EW, Jones OAH (2018) Predicted environmental concentration and fate of the top 10 most dispensed Australian prescription pharmaceuticals. Environ Sci Pollution Res Int 25(11):10966–10976. https://doi.org/10.1007/s11356-018-1343-5
Ortiz de García S, Pinto Pinto G, García Encina P, Irusta Mata R (2013) Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci Total Environ 444:451–465. https://doi.org/10.1016/j.scitotenv.2012.11.057
Östman M, Lindberg RH, Fick J, Björn E, Tysklind M (2017) Screening of biocides, metals and antibiotics in Swedish sewage sludge and wastewater. Water Res 115:318–328. https://doi.org/10.1016/j.watres.2017.03.011
Perazzolo C, Morasch B, Kohn T, Magnet A, Thonney D, Chèvre N (2010) Occurrence and fate of micropollutants in the Vidy Bay of Lake Geneva, Switzerland. Part I: priority list for environmental risk assessment of pharmaceuticals. Environ Toxicol Chem 29(8):1649–1657. https://doi.org/10.1002/etc.221
Pereira AMPT, Silva LJG, Lino CM, Meisel LM, Pena A (2017) A critical evaluation of different parameters for estimating pharmaceutical exposure seeking an improved environmental risk assessment. Sci Total Environ 603-604:226–236. https://doi.org/10.1016/j.scitotenv.2017.06.022
Petrie B, Barden R, Kasprzyk-Hordern B (2015) A review on emerging contaminants in wastewaters and the environment: current knowledge, understudied areas and recommendations for future monitoring. Water Res 72:3–27. https://doi.org/10.1016/j.watres.2014.08.053
Pubchem (2019) PubChem Database. Ibuprofen, CID=3672. Edited by National Center for Biotechnology Information. Rockville Pike, Bethesda, MD. Available online at https://pubchem.ncbi.nlm.nih.gov/compound/3672, checked on 4/9/2019
Qi C, Huang J, Wang B, Deng S, Wang Y, Yu G (2018) Contaminants of emerging concern in landfill leachate in China. Rev Emerg Contam 4(1):1–10. https://doi.org/10.1016/j.emcon.2018.06.001
Radjenović J, Petrović M, Barceló D (2009) Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res 43(3):831–841. https://doi.org/10.1016/j.watres.2008.11.043
Raju G, Sarkar P, Singla E, Singh H, Sharma RK (2016) Comparison of environmental sustainability of pharmaceutical packaging. Perspective Sci 8:683–685. https://doi.org/10.1016/j.pisc.2016.06.058
Razza F, Degli IF, Dobon A, Aliaga C, Sanchez C, Hortal M (2015) Environmental profile of a bio-based and biodegradable foamed packaging prototype in comparison with the current benchmark. J Clean Prod 102:493–500. https://doi.org/10.1016/j.jclepro.2015.04.033
Sherman J, Le C, Lamers V, Eckelman M (2012) Life cycle greenhouse gas emissions of anesthetic drugs. Anesth Analg 114(5):1086–1090. https://doi.org/10.1213/ANE.0b013e31824f6940
Siegert M-W, Lehmann A, Emara Y, Finkbeiner M (2018) Harmonized rules for future LCAs on pharmaceutical products and processes. Int J Life Cycle Assess 15(6):1542–1057. https://doi.org/10.1007/s11367-018-1549-2
Smook TM, Zho H, Zytner RG (2008) Removal of ibuprofen from wastewater: comparing biodegradation in conventional, membrane bioreactor, and biological nutrient removal treatment systems. Water Sci Technol 57(1):1–8. https://doi.org/10.2166/wst.2008.658
Stevens-Garmon J, Drewes JE, Khan SJ, McDonald JA, Dickenson ERV (2011) Sorption of emerging trace organic compounds onto wastewater sludge solids. Water Res 45(11):3417–3426. https://doi.org/10.1016/j.watres.2011.03.056
Struijs J (2013) Evaluation of the model SimpleTreat. Edited by National Institute for Public Health and the Environment. Bilthoven. Available online at https://www.rivm.nl/bibliotheek/rapporten/607105001.pdf, checked on 1/15/2019
Struijs J (2014) SimpleTreat 4.0: a model to predict fate and emission of chemicals in wastewater treatment plants. Background report describing the equations. Edited by National Institute for Public Health and the Environment. Bilthoven. Available online at https://rivm.openrepository.com/rivm/bitstream/10029/557044/3/601353005.pdf, checked on 1/10/2019
Thai PK, Jiang G, Gernjak W, Yuan Z, Lai FY, Mueller JF (2014) Effects of sewer conditions on the degradation of selected illicit drug residues in wastewater. Water Res 48:538–547. https://doi.org/10.1016/j.watres.2013.10.019
Tiwari B, Sellamuthu B, Ouarda Y, Drogui P, Tyagi RD, Buelna G (2017) Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour Technol 224:1–12. https://doi.org/10.1016/j.biortech.2016.11.042
UBA (2015) Application of SimpleTreat 4.0 in European substance regulations. Edited by Umweltbundesamt. Dessau-Roßlau. Available online at https://www.umweltbundesamt.de/sites/default/files/medien/378/publikationen/texte_13_2015_application_of_simple_treat_4.0.pdf, checked on 1/15/2019
UBA (2018a) Abfallaufkommen. Edited by Umweltbundesamt. Dessau-Roßlau. Available online at https://www.umweltbundesamt.de/daten/ressourcen-abfall/abfallaufkommen#textpart-6, checked on 1/16/2019
UBA (2018b) Ablagerungsquoten der Hauptabfallströme. Edited by Umweltbundesamt. Dessau-Roßlau. Available online at https://www.umweltbundesamt.de/daten/ressourcen-abfall/ablagerungsquoten-der-hauptabfallstroeme#textpart-1, checked on 4/8/2019
US EPA (2012) Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.11. Edited by United States Environmental Protection Agency. Washington, DC. Available online at https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface, checked on 1/16/2019
VDI (2015) Ressourceneffiziente Wasserkonzepte für Krankenhäuser. VDI ZRE Publikationen: Kurzanalyse Nr. 11. Edited by VDI Zentrum Ressourceneffizienz. Verein Deutscher Ingenieure. Berlin. Available online at https://www.ressource-deutschland.de/fileadmin/user_upload/downloads/kurzanalysen/2015-Kurzanalyse-11-VDI-ZRE-Krankenhaeuser.pdf, checked on 1/10/2019
Voigt (2018) Disposal of unused pharmaceuticals in hospitals. E-Mail (19.07.2018) to Marc-William Siegert. Berlin, 2018
de Voogt P, Janex-Habibi M-L, Sacher F, Puijker L, Mons M (2009) Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci Technol 59(1):39–46. https://doi.org/10.2166/wst.2009.764
WHO (2011) Pharmaceuticals in drinking-water. Edited by World Health Organization. Geneva. Available online at https://www.who.int/water_sanitation_health/publications/2011/pharmaceuticals_20110601.pdf, checked on 12/6/2018
WidO (2018) Anatomisch-therapeutischchemische Klassifikation mit Tagesdosen für den deutschen Arzneimittelmarkt gemäß §73 Abs. 8 Satz 5 SGB V. Beschlussfassung der Arbeitsgruppe ATC/DDD des Kuratoriums für Fragen der Klassifikation im Gesundheitswesen. Edited by Wissenschaftliches Institut der AOK. Available online at https://www.wido.de/…/wido_arz_amtl_atc_beschlussfassung_2018_1218.pdf, checked on 7/5/2019
Winker M, Faika D, Gulyas H, Otterpohl R (2008) A comparison of human pharmaceutical concentrations in raw municipal wastewater and yellowwater. Science Total Environ 399(1–3):96–104. https://doi.org/10.1016/j.scitotenv.2008.03.027
WWAP (2017) The United Nations World Water Development Report 2017. Wastewater: the untapped resource. Edited by UNESCO. Paris. Available online at https://reliefweb.int/report/world/2017-un-world-water-development-report-wastewater-untapped-resource, checked on 1/15/2019
Zampori L, Dotelli G (2014) Design of a sustainable packaging in the food sector by applying LCA. Int J Life Cycle Assess 19(1):206–217. https://doi.org/10.1007/s11367-013-0618-9
Acknowledgements
This publication is part of a project within the initiative for sustainable pharmacy, funded by the German Federal Environmental Foundation (Deutsche Bundesstiftung Umwelt, DBU). We gratefully acknowledge the engagement of the accompanying group of experts as well as the provided financial support by the DBU.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible editor: Melissa Bilec
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original version of this article unfortunately contained a mistake which was missed during typesetting. In Tab. 3, the first parameter of Flow #4 was incorrect.
Rights and permissions
About this article
Cite this article
Siegert, MW., Lehmann, A., Emara, Y. et al. Addressing the use and end-of-life phase of pharmaceutical products in life cycle assessment. Int J Life Cycle Assess 25, 1436–1454 (2020). https://doi.org/10.1007/s11367-019-01722-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11367-019-01722-7