Abstract
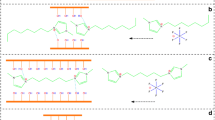
A raw illite-smectite mixed-layered clay (RI/S) was ground for preparing nano-sized I/S clay (NI/S) and subsequently amino-functionalized via grafting of 3-aminopropyltrithoxysilane (APTES) (NH2-RI/S and NH2-NI/S, respectively). The samples were characterized by particle size analysis, specific surface area measurement, X-ray diffraction (XRD), scanning electron microscopy (SEM), thermogravimetric analysis (TGA), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), and 29Si nuclear magnetic resonance (29Si NMR). Compared to RI/S, NI/S has a narrow particle size distribution and appears in a platelet-like morphology due to the disintegration/exfoliation of RI/S after grinding. Based on the 29Si NMR spectra, the appearances of tri-silicate units indicate the chemically grafting of APTES molecules on NH2-RI/S and NH2-NI/S, respectively. NH2-NI/S can adsorb greater amounts of Pb(II) cations and Cr(VI) anions rather than NH2-RI/S since NH2-NI/S grafts more amounts of amine groups (-NH2). The isotherm data for adsorption of Pb(II) cations and Cr(VI) anions can be described by the Langmuir model at different temperatures (i.e., 10 °C, 30 °C, and 50 °C), respectively. The maximum adsorption amounts of Pb(II) cations and Cr(VI) anions onto NH2-NI/S calculated by the Langmuir isotherm model are 131.23 mg/g and 36.91 mg/g at 50 °C, respectively. The adsorptions of Pb(II) cations and Cr(VI) anions onto NH2-NI/S involve in the surface complexation of NI/S and amine groups.











Similar content being viewed by others
References
Ai Z, Cheng Y, Zhang L, Qiu J (2008) Efficient removal of Cr (VI) from aqueous solution with Fe@Fe2O3 Core−Shell nanowires. Environ Sci Technol 42:6955–6960
Asgari M, Abouelmagd A, Sundararaj U (2017) Silane functionalization of sodium montmorillonite nanoclay and its effect on rheological and mechanical properties of HDPE/clay nanocomposites. Appl Clay Sci 146:439–448
Asgari M, Sundararaj U (2018) Silane functionalization of sodium montmorillonite nanoclay: the effect of dispersing media on intercalation and chemical grafting. Appl Clay Sci 153:228–238
Aytas S, Yurtlu M, Donat R (2009) Adsorption characteristic of U (VI) ion onto thermally activated bentonite. J Hazard Mater 172:667–674
Bekri-Abbes I, Srasra E (2016) Effect of mechanochemical treatment on structure and electrical properties of montmorillonite. J Alloys Compd 671:34–42
Bertuoli PT, Piazza D, Scienza LC, Zattera AJ (2014) Preparation and characterization of montmorillonite modified with 3-aminopropyltriethoxysilane. Appl Clay Sci 87:46–51
Borges R, Prevot V, Forano C, Wypych F (2017) Design and kinetic study of sustainable potential slow-release fertilizer obtained by Mechanochemical activation of clay minerals and potassium Monohydrogen phosphate. Ind Eng Chem Res 56:708–716
Cao W, Wang Z, Zeng Q, Shen C (2016) 13C NMR and XPS characterization of anion adsorbent with quaternary ammonium groups prepared from rice straw, corn stalk and sugarcane bagasse. Appl Surf Sci 389:404–410
Carneiro PA, Umbuzeiro GA, Oliveira DP, Zanoni MVB (2010) Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J Hazard Mater 174:694–699
Cataldo S, Lazzara G, Massaro M, Muratore N, Pettignano A, Riela S (2018) Functionalized halloysite nanotubes for enhanced removal of lead (II) ions from aqueous solutions. Appl Clay Sci 156:87–95
Chen W, Zhang X, Mamadiev M, Zhao C, Wang Z, Xu H (2017) Synthesis of interstratified graphene/montmorillonite composite material through organics-pillared, delamination and co-stacking and its application in hexavalent chromium removal from aqueous solution. Adv Powder Technol 28:521–533
Dambies L, Guimon C, Yiacoumi S, Guibal E (2001) Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Colloids Surf A Physicochem Eng Asp 177:203–214
Dinda D, Gupta A, Saha SK (2013) Removal of toxic Cr (VI) by UV-active functionalized graphene oxide for water purification. J Mater Chem A 1:11221–11228
Fan C, Li K, Li J, Ying D, Wang Y, Jia J (2017) Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/CoFe2O4 particles. J Hazard Mater 326:211–220
Fang F, Kong L, Huang J, Wu S, Zhang K, Wang X, Sun B, Jin Z, Wang J, Huang XJ, Liu J (2014) Removal of cobalt ions from aqueous solution by an amination graphene oxide nanocomposite. J Hazard Mater 270:1–10
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Gatti MN, Fernández LG, Sánchez MP, Parolo ME (2016) Aminopropyltrimethoxysilane- and aminopropyltrimethoxysilane-silver-modified montmorillonite for the removal of nitrate ions. Environ Technol 37:2658–2668
He C, Yang Z, Ding J, Chen Y, Tong X, Li Y (2017) Effective removal of Cr (VI) from aqueous solution by 3-aminopropyltriethoxysilane-functionalized graphene oxide. Colloids Surf A Physicochem Eng Asp 520:448–458
He H, Cao J, Duan N (2018) Novel bead-milling mechanically pulverized bulk mineral particles to ultrafine scale: energy storage and cleaner promotion of mineral extraction. J Clean Prod 198:46–53
Herrera NN, Letoffe JM, Putaux JL, David L, Bourgeat-Lami E (2004) Aqueous dispersions of silane-functionalized laponite clay platelets A first step toward the elaboration of water-based polymer/clay nanocomposites. Langmuir 20:1564–1571
Herrera NN, Letoffe JM, Reymond JP, Bourgeat-Lami E (2005) Silylation of laponite clay particles with monofunctional and trifunctional vinyl alkoxysilanes. J Mater Chem 15:863–871
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hrachová J, Komadel P, Fajnor VŠ (2007a) The effect of mechanical treatment on the structure of montmorillonite. Mater Lett 61:3361–3365
Hrachová J, Madejová J, Billik P, Komadel P, Fajnor VŠ (2007b) Dry grinding of ca and octadecyltrimethylammonium montmorillonite. J Colloid Interface Sci 316:589–595
Huang J, Ye M, Qu Y, Chu L, Chen R, He Q, Xu D (2012) Pb (II) removal from aqueous media by EDTA-modified mesoporous silica SBA-15. J Colloid Interface Sci 385:137–146
Huang Z, Li Y, Chen W, Shi J, Zhang N, Wang X, Li Z, Gao L, Zhang Y (2017) Modified bentonite adsorption of organic pollutants of dye wastewater. Mater Chem Phys 202:266–276
Jesionowski T, Krysztafkiewicz A (2001) Influence of silane coupling agents on surface properties of precipitated silicas. Appl Surf Sci 172:18–32
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Lee Y-C, Kuo C-L, Wen S-B, Lin C-P (2007) Changes of organo-montmorillonite by ball-milling in water and kerosene. Appl Clay Sci 36:265–270
Liang J, Li X, Yu Z, Zeng G, Luo Y, Jiang L, Yang Z, Qian Y, Wu H (2017) Amorphous MnO2 modified biochar derived from aerobically composted swine manure for adsorption of Pb(II) and Cd(II). ACS Sustain Chem Eng 5:5049–5058
Lin S, Liu L, Yang Y, Lin K (2017) Study on preferential adsorption of cationic-style heavy metals using amine-functionalized magnetic iron oxide nanoparticles (MIONPs-NH2) as efficient adsorbents. Appl Surf Sci 407:29–35
Lippmaa E, Maegi M, Samoson A, Engelhardt G, Grimmer AR (1980) Structural studies of silicates by solid-state high-resolution silicon-29 NMR. J Am Chem Soc 102:4889–4893
Liu W, Huang F, Wang Y, Zou T, Zheng J, Lin Z (2011) Recycling mg (OH)2 nanoadsorbent during treating the low concentration of Cr (VI). Environ Sci Technol 45:1955–1961
Liu X, Hu Q, Fang Z, Zhang X, Zhang B (2009) Magnetic chitosan nanocomposites: a useful recyclable tool for heavy metal ion removal. Langmuir 25:3–8
Luo W, Fukumori T, Guo B, Osseo-Asare K, Hirajima T, Sasaki K (2017) Effects of grinding montmorillonite and illite on their modification by dioctadecyl dimethyl ammonium chloride and adsorption of perchlorate. Appl Clay Sci 146:325–333
Maleki S, Karimi-Jashni A (2017) Effect of ball milling process on the structure of local clay and its adsorption performance for Ni (II) removal. Appl Clay Sci 137:213–224
Meng C, Zhikun W, Qiang L, Chunling L, Shuangqing S, Songqing H (2018) Preparation of amino-functionalized Fe3O4@mSiO2 core-shell magnetic nanoparticles and their application for aqueous Fe3+ removal. J Hazard Mater 341:198–206
Moore DM, Reynolds RC (1989): X-ray diffraction and the identification and analysis of clay minerals. University Press, Oxford, pp 210–211
Moreira MA, Ciuffi KJ, Rives V, Vicente MA, Trujillano R, Gil A, Korili SA, de Faria EH (2017) Effect of chemical modification of palygorskite and sepiolite by 3-aminopropyltriethoxisilane on adsorption of cationic and anionic dyes. Appl Clay Sci 135:394–404
Odom IE, Fowden L, Barrer RM, Tinker PB (1984): Smectite clay minerals: properties and uses. Philosophical Transactions of the Royal Society of London. Series A, Mathematical and Physical Sciences 311:391–409
Partlan E, Davis K, Ren Y, Apul OG, Mefford OT, Karanfil T, Ladner DA (2016) Effect of bead milling on chemical and physical characteristics of activated carbons pulverized to superfine sizes. Water Res 89:161–170
Petra L, Billik P, Melichová Z, Komadel P (2017) Mechanochemically activated saponite as materials for Cu2+ and Ni2+ removal from aqueous solutions. Appl Clay Sci 143:22–28
Piscitelli F, Posocco P, Toth R, Fermeglia M, Pricl S, Mensitieri G, Lavorgna M (2010) Sodium montmorillonite silylation: unexpected effect of the aminosilane chain length. J Colloid Interface Sci 351:108–115
Puchana-Rosero MJ, Adebayo MA, Lima EC, Machado FM, Thue PS, Vaghetti JCP, Umpierres CS, Gutterres M (2016) Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf A Physicochem Eng Asp 504:105–115
Ramadan AR, Esawi AMK, Gawad AA (2010) Effect of ball milling on the structure of Na+-montmorillonite and organo-montmorillonite (Cloisite 30B). Appl Clay Sci 47:196–202
Reynolds RC (1992) X-ray diffraction studies of Illite/Smectite from rocks, < 1 μm randomly oriented powders, and < 1 μm oriented powder aggregates: the absence of laboratory-induced artifacts. Clay Clay Miner 40:387–396
Saha B, Orvig C (2010) Biosorbents for hexavalent chromium elimination from industrial and municipal effluents. Coord Chem Rev 254:2959–2972
Shanmugharaj AM, Rhee KY, Ryu SH (2006) Influence of dispersing medium on grafting of aminopropyltriethoxysilane in swelling clay materials. J Colloid Interface Sci 298:854–859
Sun K, Shi Y, Wang X, Rasmussen J, Li Z, Zhu J (2017) Organokaolin for the uptake of pharmaceuticals diclofenac and chloramphenicol from water. Chem Eng J 330:1128–1136
Suraj G, Iyer CSP, Rugmini S, Lalithambika M (1997) The effect of micronization on kaolinites and their sorption behaviour. Appl Clay Sci 12:111–130
Tang N, Niu C-G, Li X-T, Liang C, Guo H, Lin L-S, Zheng C-W, Zeng G-M (2018) Efficient removal of Cd2+ and Pb2+ from aqueous solution with amino- and thiol-functionalized activated carbon: isotherm and kinetics modeling. Sci Total Environ 635:1331–1344
Tao Q, Su L, Frost RL, Zhang D, Chen M, Shen W, He H (2016) Silylation of mechanically ground kaolinite. Clay Miner 49:501–608
Thue PS, Sophia AC, Lima EC, Wamba AGN, de Alencar WS, dos Reis GS, Rodembusch FS, Dias SLP (2018) Synthesis and characterization of a novel organic-inorganic hybrid clay adsorbent for the removal of acid red 1 and acid green 25 from aqueous solutions. J Clean Prod 171:30–44
Tian Y, Li H, Liu Y, Cui G, Sun Z, Yan S (2016) Morphology-dependent enhancement of template-guided tunable polyaniline nanostructures for the removal of Cr (VI). RSC Adv 6:10478–10486
Toor M, Jin B, Dai S, Vimonses V (2015) Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J Ind Eng Chem 21:653–661
Tsai WT, Lai CW, Hsien KJ (2003) Effect of particle size of activated clay on the adsorption of paraquat from aqueous solution. J Colloid Interface Sci 263:29–34
Wamba AGN, Lima EC, Ndi SK, Thue PS, Kayem JG, Rodembusch FS, dos Reis GS, de Alencar WS (2017) Synthesis of grafted natural pozzolan with 3-aminopropyltriethoxysilane: preparation, characterization, and application for removal of brilliant green 1 and reactive black 5 from aqueous solutions. Environ Sci Pollut Res 24:21807–21820
Weiss CA Jr, Altaner SP, Kirkpatrick RJ (1987) High-resolution 29Si NMR spectroscopy of 2:1 layer silicates: correlations among chemical shift, structural distortions, and chemical variations. Am Mineral 72:935–942
Zhang H, Gu L, Zhang L, Zheng S, Wan H, Sun J, Zhu D, Xu Z (2017) Removal of aqueous Pb (II) by adsorption on Al2O3-pillared layered MnO2. Appl Surf Sci 406:330–338
Zhang J, Yan Z, Ouyang J, Yang H, Chen D (2018a) Highly dispersed sepiolite-based organic modified nanofibers for enhanced adsorption of Congo red. Appl Clay Sci 157:76–85
Zhang S, Wang Z, Chen H, Kai C, Jiang M, Wang Q, Zhou Z (2018b) Polyethylenimine functionalized Fe3O4/steam-exploded rice straw composite as an efficient adsorbent for Cr (VI) removal. Appl Surf Sci 440:1277–1285
Funding
This work was supported by the Major Scientific and Technological Projects of Guangdong Province, China (No. 2015B090927002), the Fundamental Research Funds for the Central Universities (No. 2015ZM102).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Tito Roberto Cadaval Jr
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Nano-sized illite-smectite (NI/S) clay was prepared in a high-energy density stirred bead mill.
• NI/S was amino-functionalized via grafting of 3-aminopropyltrithoxysilane (NH2-NI/S).
• Amino-functionalization enhanced the adsorption amounts of Pb(II) cations and Cr(VI) anions in aqueous solution.
Electronic supplementary material
ESM 1
(DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Pan, Z. & Wang, Y. Enhanced adsorption of cationic Pb(II) and anionic Cr(VI) ions in aqueous solution by amino-modified nano-sized illite-smectite clay. Environ Sci Pollut Res 26, 11126–11139 (2019). https://doi.org/10.1007/s11356-019-04447-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-04447-0