Abstract
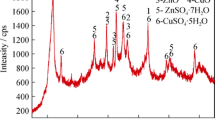
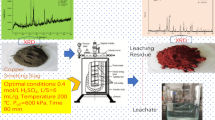
To comprehensively reuse the leaching residue obtained from lead-zinc tailings, an active silicon adsorbent (ASA) was prepared from leaching residue and studied as an adsorbent for copper(II), lead(II), zinc(II), and cadmium(II) in this paper. The ASA was prepared by roasting the leaching residue with either a Na2CO3/residue ratio of 0.6:1 at 700 °C for 1 h or a CaCO3/residue ratio of 0.8:1 at 800 °C for 1 h. Under these conditions, the available SiO2 content of the ASA was more than 20%. The adsorption behaviors of the metal ions onto the ASA were investigated and the Langmuir, Freundlich, and Dubinin-Radushkevich isotherm models were used to analyze the adsorption isotherm. The result showed that the maximum adsorption capacities of copper(II), lead(II), cadmium(II), and zinc(II) calculated by the Langmuir model were 3.40, 2.83, 0.66, and 0.62 mmol g−1, respectively. The FT-IR spectra of the ASA and the mean free adsorption energies indicated that ion exchange was the mechanism of copper(II), lead(II), and cadmium(II) adsorption and that chemical reaction was the mechanism of zinc(II) adsorption. These results provide a method for reusing the leaching residue obtained from lead-zinc tailings and show that the ASA is an effective adsorbent for heavy metal pollution remediation.












Similar content being viewed by others
References
Alter A, Wiech G (1978) X-ray spectroscopic studies on the electronic structure of binary glasses. Jpn J Appl Phys Suppl 17:288–290
Anbia M, Kargosha K, Khoshbooei S (2015) Heavy metal ions removal from aqueous media by modified magnetic mesoporous silica MCM-48. Chem Eng Res Des 93:779–788
Andrejkovičová S, Sudagar A, Rocha J, Patinha C, Hajjaji W, Da Silva EF, Velosa A, Rocha F (2016) The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers. Appl Clay Sci 126:141–152
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Bhattacharyya KG, Sen Gupta S (2006) Pb(II) uptake by kaolinite and montmorillonite in aqueous medium: influence of acid activation of the clays. Colloids Surf A Physicochem Eng Asp 277:191–200
Duan Q, Lee J, Liu Y, Chen H, Hu H (2016) Distribution of heavy metal pollution in surface soil samples in China: a graphical review. Bull Environ Contam Toxicol 97:303–309
Erdem E, Karapinar N, Donat R (2004) The removal of heavy metal cations by natural zeolites. J Colloid Interface Sci 280:309–314
Fan X, Wen X, Huang F, Cai Y, Cai K (2016) Effects of silicon on morphology, ultrastructure and exudates of rice root under heavy metal stress. Acta Physiol Plant 38:197
Figueira P, Lopes CB, Daniel-da-Silva AL, Pereira E, Duarte AC, Trindade T (2011) Removal of mercury (II) by dithiocarbamate surface functionalized magnetite particles: application to synthetic and natural spiked waters. Water Res 45:5773–5784
Gao J, Zhong SH (2001) Supported dicopper(II) complex catalyst Cu2(II)(m-Br)2/SiO2: synthesis, characterization and catalytic properties for synthesis of ethylene carbonate. Appl Catal A Gen 220:1–8
Gu HH, Qiu H, Tian T, Zhan SS, Deng THB, Chaney RL, Wang SZ, Tang YT, Morel JL, Qiu RL (2011) Mitigation effects of silicon rich amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on multi-metal contaminated acidic soil. Chemosphere 83:1234–1240
Gyollai I, Krebsz M, Kereszturi Á, Bérczi S, Gucsik A (2010) FTIR-ATR spectroscopy of shock vein in Mócs L6 chondrite. J Geophys Res 116:E08010
Hamon RE, McLaughlin MJ, Cozens G (2002) Mechanisms of attenuation of metal availability in in situ remediation treatments. Environ Sci Technol 36:3991–3996
Heidari A, Younesi H, Mehraban Z (2009) Removal of Ni(II), Cd(II), and Pb(II) from a ternary aqueous solution by amino functionalized mesoporous and nano mesoporous silica. Chem Eng J 153:70–79
Hu C, Zhu P, Cai M, Hu H, Fu Q (2017) Comparative adsorption of Pb(II), Cu(II) and Cd(II) on chitosan saturated montmorillonite: kinetic, thermodynamic and equilibrium studies. Appl Clay Sci 143:320–326
Kilislioglu A, Bilgin B (2003) Thermodynamic and kinetic investigation of uranium adsorption on amberlite IR-118-H resin. Appl Radiat Isot 50:155–160
Lei C, Yan B, Chen T, Quan S-X, Xiao X-M (2015) Comprehensive utilization of lead–zinc tailings, part 1: pollution characteristics and resource recovery of sulfur. J Environ Chem Eng 3:862–869
Lei C, Yan B, Chen T, Xiao XM (2017) Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation. J Clean Prod 158:73–80
Lei C, Yan B, Chen T, Wang X-L, Xiao X-M (2018) Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J Clean Prod 181:408–415
Liang X, Xu Y, Sun G, Wang L, Sun Y, Qin X (2009) Preparation, characterization of thiol-functionalized silica and application for sorption of Pb2+ and Cd2+. Colloids Surf A Physicochem Eng Asp 349:61–68
Liu Y, Fu R, Sun Y, Zhou X, Baig SA, Xu X (2016) Multifunctional nanocomposites Fe3O4@SiO2-EDTA for Pb(II) and Cu(II) removal from aqueous solutions. Appl Surf Sci 369:267–276
Ma JF, Yamaji N (2006) Silicon uptake and accumulation in higher plants. Trends Plant Sci 11:392–397
Monika FM, Baccaro S, Sharma G, Thind KS, Singh DP (2012) Role of aluminium oxide in the structure of heavy metal oxide borosilicate glasses. Phys Status Solidi 209:1438–1444
Naseem R, Tahir S (2001) Removal of Pb (II) from aqueous/acidic solutions by using bentonite as an adsorbent. Water Res 35:3982–3986
Neumann D, Zur Nieden U (2001) Silicon and heavy metal tolerance of higher plants. Phytochemistry 56:685–692
Nguyen TC, Loganathan P, Nguyen TV, Vigneswaran S, Kandasamy J, Naidu R (2015) Simultaneous adsorption of Cd, Cr, Cu, Pb, and Zn by an iron-coated Australian zeolite in batch and fixed-bed column studies. Chem Eng J 270:393–404
Ning D, Liang Y, Liu Z, Xiao J, Duan A (2016) Impacts of steel-slag-based silicate fertilizer on soil acidity and silicon availability and metals-immobilization in a Paddy soil. PLoS One 11:e0168163
Richmond KE, Sussman M (2003) Got silicon? The non-essential beneficial plant nutrient. Curr Opin Plant Biol 6:268–272
Rodríguez Martín JA, De Arana C, Ramos-Miras JJ, Gil C, Boluda R (2015) Impact of 70 years urban growth associated with heavy metal pollution. Environ Pollut 196:156–163
Sakoda T, Oda M (1969) Spectrophotometric determination of silicon in white cast iron with molybdenum blue method. Bunseki Kagaku 18:256–258
Shim J, Shea PJ, Oh BT (2014) Stabilization of heavy metals in mining site soil with silica extracted from corn cob. Water Air Soil Pollut 225:1–12
Tang F-J, Qu H-J, Zhang Z-Y (2006) Summary about the silicon fertilizer production. J Heilongjiang Aug First Land Reclamation Univ 4:018
Tatzber M, Stemmer M, Spiegel H, Katzlberger C, Haberhauer G, Gerzabek M (2007) An alternative method to measure carbonate in soils by FT-IR spectroscopy. Environ Chem Lett 5:9–12
Unlü N, Ersoz M (2006) Adsorption characteristics of heavy metal ions onto a low cost biopolymeric sorbent from aqueous solutions. J Hazard Mater 136:272–280
US E (1996): Method 3052: microwave assisted and digestion of siliceous and organically based matrices, Washington, DC
Yoon MY, Lee S, Choo JH, Jang H, Cho W, Kang H, Park J-K (2016) Economical synthesis of complex silicon fertilizer by unique technology using loess. Korean J Chem Eng 33:958–963
Zheng H, Han L, Ma H, Zheng Y, Zhang H, Liu D, Liang S (2008) Adsorption characteristics of ammonium ion by zeolite 13X. J Hazard Mater 158:577–584
Zolfaghari G, Esmaili-Sari A, Anbia M, Younesi H, Amirmahmoodi S, Ghafari-Nazari A (2011) Taguchi optimization approach for Pb(II) and Hg(II) removal from aqueous solutions using modified mesoporous carbon. J Hazard Mater 192:1046–1055
Acknowledgements
This work was supported by Guangdong provincial science and technology program (No. 2014B090901040, 2015B090922005), National Natural Science Foundation of China (No. 41503116), Guangdong Natural Science Funds for Distinguished Young Scholar (No. S2013050014122), “Guangdong Te Zhi program” youth science and technology talent of project (No. 2014TQ01Z262), and Guangzhou science and technology program (201607020003). This is contribution No. IS-2536 from GIGCAS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Guilherme L. Dotto
Rights and permissions
About this article
Cite this article
Lei, C., Yan, B., Chen, T. et al. Preparation and adsorption characteristics for heavy metals of active silicon adsorbent from leaching residue of lead-zinc tailings. Environ Sci Pollut Res 25, 21233–21242 (2018). https://doi.org/10.1007/s11356-018-2194-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2194-9